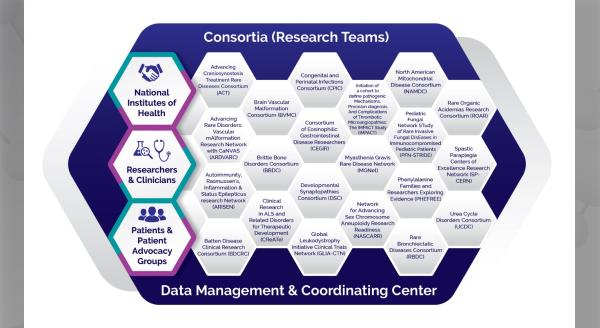
On June 21, 2016, the NIH announced a mandate requiring the use of a single Institutional Review Board (sIRB) for multi-site, federally-funded studies, with a compliance date of September 25, 2017. The intent of the mandate is to increase efficiency, uniformity, and reduce the time to obtain IRB approval for multiple sites on the premise that individual sites submitting research to their local IRBs is costly, results in duplication of effort, results in inconsistencies among sites, and delays implementation of research.
As a result, many institutions and researchers are working to figure out how to comply with the mandate. After all, many IRBs do not have established processes in place for relying on other IRBs or for serving as the IRB of record for multiple sites. Although sIRB review has been permitted under the regulations since 1991, many researchers and IRBs have not actually used this method of IRB review.
This is a time for IRBs and researchers who have utilized sIRB review to share their experiences, information, and lessons learned with others. During the Rare Diseases Clinical Research Network (RDCRN) Steering Committee meeting held on November 4, 2016, a group of panelists provided their insight and experiences regarding the use of an sIRB for multi-site research. The panelists provided their experiences from the perspectives of the IRB of record, the research site, and the Consortium Principal Investigator (PI).
In his presentation titled, “Conducting Research While Living with a Single IRB,” James E. McNerney, JD, CIP, RAC, Central IRB Liaison in the Children’s National Medical Center Office for Protection of Human Subjects (OPHS), provided a historical perspective on the implementation of an sIRB for the Urea Cycle Disorders Consortium (UCDC). When the UCDC set out to begin using an sIRB in 2014, it planned to do so for three ongoing studies. New studies that would be developed in the future would also be submitted to the sIRB. To facilitate the implementation process, the OPHS created the position of a Senior Regulatory Specialist/Central IRB Liaison to manage sIRB operations and to interface with sites.
The sIRB was charged with determining the optimum way to transfer the three ongoing studies from the local IRBs, where they were already approved, to the sIRB without experiencing any lapses in IRB approval, interruptions in research activities at the sites, and maintaining a clean audit trail. One of the main challenges faced by the single IRB was developing a level of trust between the relying institutions, study teams, and the sIRB.
Mr. McNerney found it helpful to have the support of the lead PI and his institution and to utilize the lead site as the clearinghouse for all sIRB actions. He also found it beneficial to have a dedicated central IRB liaison knowledgeable in both study conduct and IRB administration.
Overall, Mr. McNerney stressed that while it is more complicated to transition ongoing studies from local IRBs to an sIRB than it is to submit new studies to the sIRB, it can be done successfully. He emphasized the need to establish open lines of communication between all parties early in the process and added that a climate of cooperation is also important.
Kara L. Simpson, MS, CGC, certified genetic counselor in the Division of Genetics and Metabolism at Children’s National Health System, provided information from the site level perspective. She reviewed the process for submitting IRB applications to the sIRB for initial review, amendment reviews, and continuing reviews. After going through the process of sIRB review, UCDC sites were surveyed. Of those that responded, 8/13 (62%) indicated that it was a smooth process to transition a study from the local IRB to the sIRB. Another 3/13 (23%) responded that it was a difficult process and 2/13 (15%) were indifferent. Sites reported most of the difficulties to be related to their local IRB, delays in obtaining sIRB-approved consent forms translated into other languages, and confusion over local IRB versus sIRB oversight. Because materials were submitted on behalf of sites to the sIRB, as opposed to those sites submitting materials to the IRB themselves, some sites felt they were less familiar with the study and IRB status. In the end, sites reported the following as advantages of using an sIRB: saves time, is easier, increased uniformity among sites, and improved language in informed consent forms.
To provide the perspective of the PI, Marc Rothenberg, MD, PhD, Consortium PI for the Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR) and director of the Division of Allergy and Immunology and the Cincinnati Center for Eosinophilic Disorders at Cincinnati Children’s Hospital Medical Center, relayed the experience of CEGIR. He explained the process of sIRB review from obtaining reliance agreements to study activation. Dr. Rothenberg echoed some of the same points made by Mr. McNerney. He stressed the importance of the support of the institutions involved, the need for dedicated personnel to lead the sIRB review process, and the need for patience. While the process of sIRB review is initially very time consuming and requires a tremendous amount of work, Dr. Rothenberg said the use of an sIRB resulted in uniformity among sites and streamlined approvals for amendments and continuing reviews.
While there are advantages to using an sIRB, establishing one often poses challenges for the researchers and the institutions involved. However, there are some recent initiatives that have been developed to facilitate sIRB review.
Dr. Nichelle Cobb discussed one such initiative, the SMART IRB Reliance Platform, which is funded by the NIH Clinical and Translational Science Awards (CTSA) Program and led by Harvard Catalyst, University of Wisconsin ICTR, and Dartmouth Synergy. Smart IRB provides flexible resources that investigators nationwide can use to harmonize and streamline IRB review for their own multi-site studies. The model includes a flexible master IRB reliance agreement and standard operating procedures to clarify roles, responsibilities, and processes for single IRB review. Dr. Cobb illustrated and reported outcomes from the INVESTED study’s use of the SMART IRB Agreement as a testing model for IRB reliance among 14 sites.
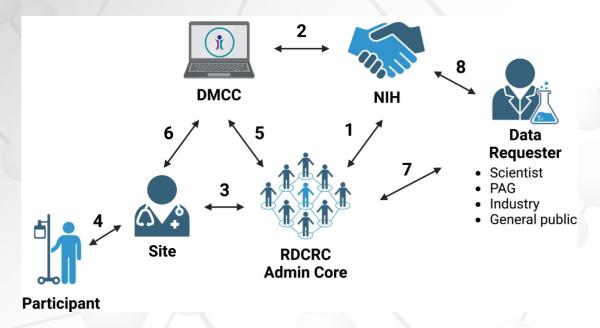
The OneIRB Coordinating Center, developed by the Health Informatics Institute at the University of South Florida, is another initiative that provides tools and coordinating center support to study teams and IRBs to assist in the implementation of sIRB review. OneIRB provides a reliance agreement template which covers all studies conducted by a consortium or network, along with forms for local context review, continuing review and reportable events, which can be tailored to meet the requirements of the sIRB. The Smart IRB master agreement can be used in conjunction with OneIRB.
Just as Mr. McNerney utilized the lead site as the clearinghouse for all sIRB actions, so do other consortia using an sIRB. To relieve this burden on the lead site, OneIRB includes a coordinating center component which serves as the clearinghouse for documents going to and from sites and the sIRB. OneIRB distributes the reliance agreement and local context review forms to relying institutions, distributes informed consent form templates to sites for insertion of site-specific language, and obtains site delegation logs and site-specific recruitment materials from sites. OneIRB then completes the submission to the sIRB on behalf of sites and distributes sIRB approvals once they are obtained. OneIRB currently works with over 100 sites and 20 protocols for RDCRN consortia as well as the Type 1 Diabetes TrialNet group of researchers.