Data Management and Coordinating Center
The DMCC at Cincinnati Children’s Hospital Medical Center has been funded by the DRDRI at NCATS since 2019. We are currently supporting Cycle 5 of the RDCRN to facilitate Network operations, research, participant engagement, and data sharing.
Our Aims

Support the RDCRN and advance the methods and the practice of rare diseases clinical research to promote clinical trial readiness

Maintain a leading-edge, cloud-based data ecosystem to facilitate the research done by the rare diseases clinical research consortia and promote data sharing within the RDCRN and with other stakeholders

Expand research collaboration within the RDCRN and with other partners, disseminate the RDCRN research findings, and promote the RDCRN globally
Four Cores of the DMCC
Administrative Core | |
 |
|
Clinical Research Core | |
 |
|
Data Management Core | |
 |
|
Engagement and Dissemination Core | |
 |
|
RDCRN Resources Maintained by the DMCC
The DMCC offers many resources to RDCRN members. Please see examples below. Contact support@rdcrn.org with any questions.
RDCRN Protocols
The DMCC engages the consortia in developing high-quality human research protocols. Upon request by the investigators, the DMCC systematically reviews protocols and amendments before submission to the NIH, providing prompt feedback to the protocol principal investigator and to the NIH program officer.
Protocol resources include:
- Guidance on using language from grant applications to develop human research protocols
- Template protocols, including structured synopsis and schedule of events sections
- Recommended language for informed consent documents, including text to communicate data sharing with the NIH and DMCC
- Comprehensive review of protocol drafts, including regulatory aspects of protocol and informed consent, study design, aims, and data management and analysis plans
- Training materials, including slide shows and video presentations
RDCRN Tool Garden
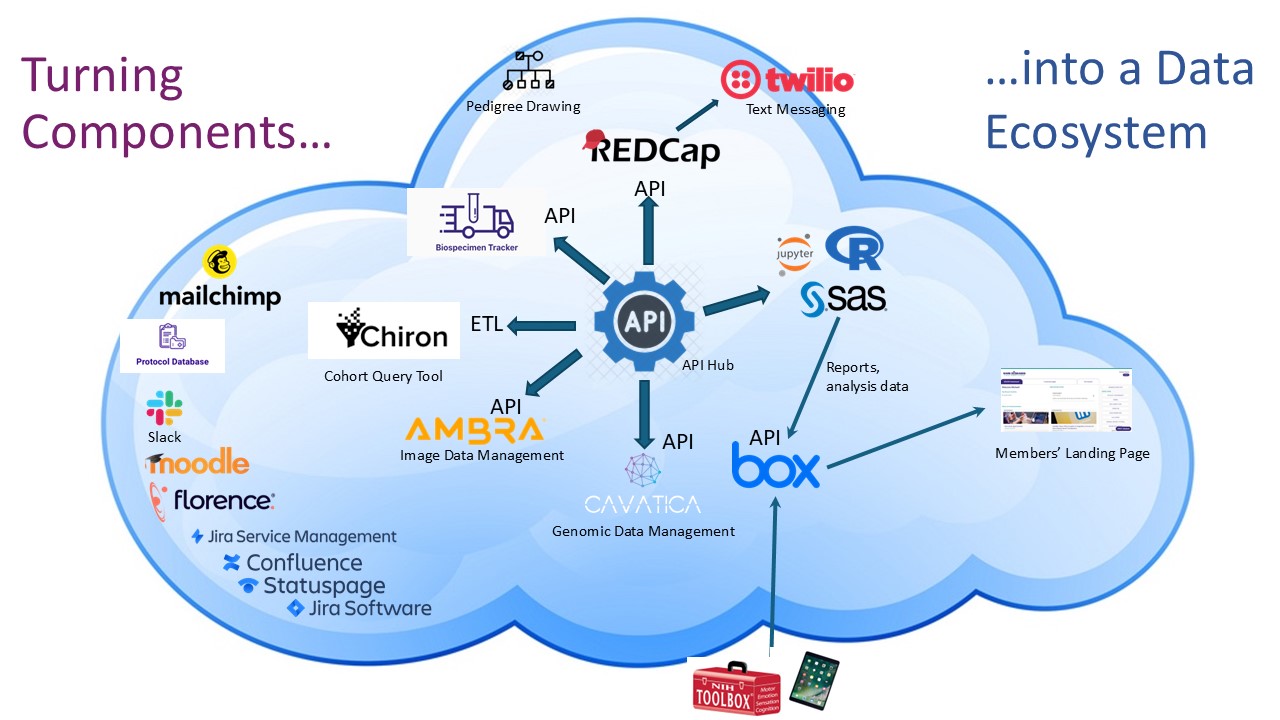
The RDCRN Cloud Environment consists of a variety of secure, web-based tools. RDCRN consortia can use these tools to assist with data management, analysis, communication, administration, and security. Consortia are responsible for all data stored in the Cloud, as well as access permissions. All tools in the Cloud can be accessed with the same single sign-on, which uses federated authentication with InCommon, the National Institutes of Health (NIH), and Login.gov. By utilizing automated data flows, the Cloud seamlessly integrates data integrity, monitoring, reporting, storage, and analysis.
Tools include:
Data Management and Analysis
- Ambra Imaging Platform—Ambra’s cloud-based platform enables medical image uploads as well as sharing via automated routing capabilities.
- Biospecimen Tracker—Allows users to view and track biosample shipments previously registered in a specially configured REDCap form.
- Box—Box is a content management platform for all RDCRN members that allows users to store and share RDCRN files securely across institutions.
- Cavatica—CAVATICA is a data analysis and sharing platform designed to accelerate discovery in a scalable, cloud-based compute environment
- Chiron—The RDCRN Data Catalog utilizes a platform called Chiron to provide an easy to use cohort query tool.
- Florence—Florence is an eRegulatory and document management platform for clinical research sites that helps study teams organize, standardize, store, access, and share regulatory documents.
- JupyterHub—JupyterHub brings the power of notebooks to groups of users.
- NIH Toolbox—The NIH Toolbox is a multidimensional set of brief measures assessing cognitive, emotional, motor and sensory function from ages 3 to 85, meeting the need for a standard set of measures that can be used as a “common currency” across diverse study designs and settings.
- Pedigree Drawing Tool—The Pedigree Drawing Tool is an application for all RDCRN members used to create, view, and edit patient and family records.
- REDCap—REDCap (Research Electronic Data Capture) is a web-based application for RDCRN members who are part of specific research protocols. It is used to capture data for clinical research, create surveys, and manage projects.
- RStudio—RStudio is an integrated development environment (IDE) for the programming language R.
- SAS Studio—SAS Studio is a web browser-based programming environment for all RDCRN members that allows writing and running SAS code.
Utility, Communication, and Administration
- Atlassian Tools
- Confluence—Confluence is a team workspace for DMCC use where RDCRN documentation, resources, user guides, and FAQs for tools, solutions, and processes are shared.
- DMCC Help Center—The DMCC Help Center is an online knowledge hub for our IT services and support that includes FAQs, how-to articles, and a contact form to ask questions, request access, or report problems.
- JIRA—JIRA is a project management tool for DMCC use that allows planning, tracking, and managing requests in the DMCC Help Center. It powers our web ticket and system access request systems.
- Drupal—Drupal is a web content management system for DMCC use that powers the RDCRN website.
- GitHub—GitHub is a development platform for DMCC use that hosts code and allows users to build software.
- Grants Portal—Grants Portal is a platform to allow RDCRN members to run their own grant competitions including pilot projects and research awards.
- Mailchimp—Mailchimp is a marketing platform for DMCC use that allows users to create, send, and track emails. We use this for the Weekly Update and the Spotlight Newsletter.
- Members Landing Page (MLP)—The RDCRN Members Landing Pages provide information and links to different RDCRN resources at different levels: network-wide, at the consortium level and at the protocol level.
- Moodle—Moodle is an online learning platform for all RDCRN members that hosts training modules for clinical research protocols. Available modules can be accessed from the Member’s Landing Page.
- Protocol Database—The Protocol Database website allows RDCRN members to view basic details around all RDCRN protocols. Select Consortia members can make edits while others can request changes.
- Slack—Slack is a messaging platform for all RDCRN members that allows connection, collaboration, and interaction with other members.
- StatusPage—The Status Page is a real-time status dashboard for all RDCRN members that shows notifications for upcoming maintenance and scheduled site outages.
- Twilio Text Messaging—Twilio is a text messaging-based communication tool available for any REDCap project that allows study teams to deliver SMS alerts, notifications, and reminders to research participants.
- Vimeo—Vimeo is a platform for DMCC use that allows users to host and share video.
- YouTube Channel—Video sharing platform for RDCRN content.
- Zoom—Zoom is a cloud-based platform for video and audio conferencing.
Security
- Duo MFA—Duo is a two-factor authentication method to verify your identity (using a phone or other mobile device) for use by anyone accessing RDCRN secure assets. It adds a second layer of security for RDCRN applications that can hold sensitive information.
- Zix Email Encryption—Zix is an automatic encryption platform for all emails sent from REDCap or the Biospecimen Tracker that ensures security and prevents data loss.
Consortium Guidance and Tools
- RDCRN Policies
- RDCRN Data Standards
- Data Sharing guidance, recommended legal instruments, recommended language for ICF, DMS, other documents finalized during cycle 4
- Requirements for dataset ingestion into RDCRN data repository
DMCC Leadership Team

Maurizio Macaluso, MD, DrPH, FACE
Contact Principal Investigator
Director, Division of Biostatistics and Epidemiology, Cincinnati Children’s Hospital Medical Center
Professor, Department of Pediatrics, University of Cincinnati College of Medicine

Michael Wagner, PhD
MPI
Associate Professor, Division of Biomedical Informatics, Cincinnati Children’s Hospital Medical Center
Associate Professor, Department of Pediatrics, University of Cincinnati College of Medicine

