Consortia Directory
The RDCRN is designed to advance medical research on rare diseases by providing support for clinical studies and facilitating collaboration, study enrollment and data sharing.
Currently, the RDCRN consists of 21 individual clinical research consortia and a Data Management and Coordinating Center (DMCC). Each consortium focuses on at least three related rare diseases or conditions, participates in multisite studies and actively involves patient advocacy groups as research partners. The DMCC enables uniform high-quality data collection and analysis, and facilitates information sharing across the network. This robust data source helps scientists better understand the common elements of rare diseases so they may apply that knowledge to improving diagnosis and treatment for these conditions.
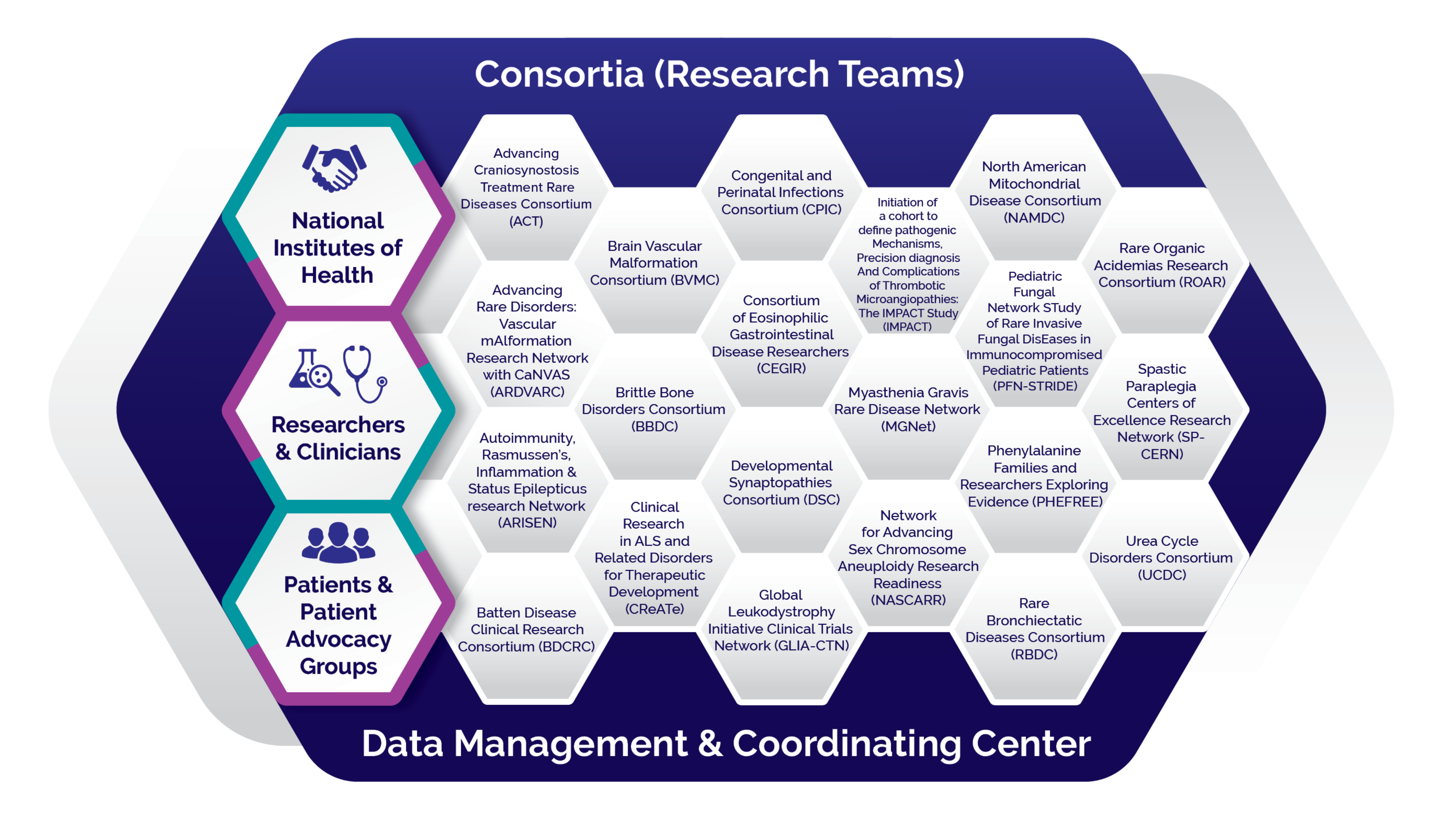
Learn More About the RDCRN Consortia
Advancing Craniosynostosis Treatment Rare Diseases Consortium
ACT
Advancing Craniosynostosis Treatment Rare Diseases Consortium
Grant number: U54 DE035976-01
Principal Investigator: Eric Chien-Wei Liao, MD, PhD
Consortium Project Manager:
Contact Information:
ACT is funded under grant number U54 DE035976 as a collaboration between NCATS, the National Institute of Dental and Craniofacial Research (NIDCR), and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD).
Advancing Rare Disorders: Vascular mAlformation Research Network with CaNVAS
ARDVARC
Advancing Rare Disorders: Vascular mAlformation Research Network with CaNVAS
Grant number: U54 HL185006-01
Principal Investigator: Denise Adams, MD | Michael Jeng, MD

Advancing Rare Disorders: Vascular mAlformation Research Network with CaNVAS (ARDVARC) builds on the established clinical trial network, CaNVAS (Consortium of iNvestigators of Vascular AnomalieS). Our goal is to strengthen research and improve care for people living with vascular malformations. By bringing together patients, families, physicians, researchers, and other health care professionals, we work to advance understanding of these rare conditions and translate discoveries into better treatments.
Consortium Project Manager: Melissa Casey, MB
Contact Information: caseyma@chop.edu
ARDVARC is funded under grant number U54HL185006 as a collaboration between NCATS, and the National Heart, Lung, and Blood Institute (NHLBI).
Autoimmunity, Rasmussen’s, Inflammation & Status Epilepticus Research Network
ARISEN
Autoimmunity, Rasmussen’s, Inflammation & Status Epilepticus Research Network
Grant number: U54 HD122209-01
Principal Investigator: Grace Gombolay, MD
The Autoimmunity, Rasmussen’s, Inflammation & Status Epilepticus Research Network (ARISEN) is a Rare Diseases Clinical Research Network (RDCRN) consortium to advance research on severe, immune-mediated neurologic disorders. ARISEN brings together multidisciplinary scientists and clinicians to accelerate diagnosis, deepen mechanistic understanding, and improve outcomes for patients affected by autoimmune encephalitis, Rasmussen’s encephalitis, and new-onset refractory status epilepticus (NORSE). As part of the RDCRN, ARISEN leverages a national collaborative infrastructure supported by NCATS, NICHD, and the Data Management and Coordinating Center to drive high-impact, patient-centered research across these rare and devastating conditions.
Consortium Project Manager: Kaci McCoy
Contact Information: Kaci.mccoy@emory.edu
ARISEN is funded under grant number U54HD122209 as a collaboration between NCATS and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD).
Batten Disease Clinical Research Consortium
BDCRC
Batten Disease Clinical Research Consortium
Grant number: U54 HD122210-01
Principal Investigator: Erika Augustine, MD, MS
Consortium Project Manager:
Contact Information:
BDCRC is funded under grant number U54HD122210 as a collaboration between NCATS, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute of Neurological Disorders and Stroke (NINDS).
Brain Vascular Malformation Consortium
BVMC
Brain Vascular Malformation Consortium
Grant number: U54 NS065705-11
Principal Investigator: Helen Kim, MPH, PhD

The Brain Vascular Malformation Consortium (BVMC) focuses on three rare brain conditions: familial cerebral cavernous malformation (CCM), Sturge-Weber syndrome (SWS), and hereditary hemorrhagic telangiectasia (HHT). These disorders are poorly understood, costly to manage, and can cause serious complications such as hemorrhages, seizures, and problems with spinal cord, nerve or brain function. Over the last ten years, the BVMC made great progress in creating patient registries that led to several important discoveries, including the gene for SWS port-wine stain, the first predictor that stratified hemorrhage risk in HHT patients with brain arteriovenous malformations, and inflammatory genetic modifiers of a novel signaling pathway involved in CCM lesion formation. These scientific discoveries in turn help doctors better diagnose and treat patients. Looking ahead to the next five years, BVMC projects will continue these important lines of work in preparation for clinical trials of treatments for these disorders, working closely with three patient advocacy groups and 24 clinical recruitment centers across North America and Europe.
Consortium Project Manager: Phillip Evans
Contact Information: phillip.evans@ucsf.edu
BVMC is funded under grant number U54NS065705 as a collaboration between NCATS and the National Institute of Neurological Disorders and Stroke (NINDS).
Brittle Bone Disorders Consortium
BBDC
Brittle Bone Disorders Consortium
Grant number: U54 AR068069-06
Principal Investigator: Brendan Lee, MD

Brittle bone disorders (BBDs), also known as osteogenesis imperfecta, includes 13 inherited conditions involving bones that break easily. Brittle bone disorders can cause deformity and chronic pain and lead to premature death. The Brittle Bone Disorders Consortium (BBDC) includes 14 medical research sites working to better understand and treat these disorders.
Consortium Project Manager: Dianne Nguyen, BS
Contact Information: diannen@bcm.edu
BBDC is funded under grant number U54AR068069 as a collaboration between NCATS, the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Institute of Dental and Craniofacial Research (NIDCR), the National Human Genome Research Institute (NHGRI), the Office of Research on Women’s Health (ORWH), and the NIH Office of the Director (OD).
Clinical Research in ALS and Related Disorders for Therapeutic Development
CReATe
Clinical Research in ALS and Related Disorders for Therapeutic Development
Grant number: U54 NS092091-06
Principal Investigator: Michael Benatar, MD, PhD

Amyotrophic lateral sclerosis (ALS) is a fatal disease that involves progressive death of motor nerves in the brain, brainstem, and spinal cord. The disease is closely related to disorders such as primary lateral sclerosis, hereditary spastic paraplegia, progressive muscular atrophy, and frontotemporal dementia. These diseases have shared genetic causes and underlying biology as well as a shared lack of effective therapies. The Clinical Research in ALS and Related Disorders for Therapeutic Development (CReATe) Consortium brings together a multidisciplinary group of researchers and a diverse array of patient advocacy groups to bridge the gap between basic scientists and investigators engaged in clinical-translational research. The goals of the CReATe Consortium are to advance understanding of the contribution of genetics to these complex disorders, promote development of biological features (biomarkers) that may enhance therapeutic development efforts, train a new generation of clinical scientists, and engage the public and scientific communities in partnerships that enhance clinical research and therapy development.
Consortium Project Manager: Anne Cooley
Contact Information: projectcreate@miami.edu
CReATe is funded under grant number U54NS092091 as a collaboration between NCATS, the National Institute of Neurological Disorders and Stroke (NINDS), and the National Institute on Aging (NIA).
Congenital and Perinatal Infections Consortium
CPIC
Congenital and Perinatal Infections Consortium
Grant number: U54 AI150225-01
Principal Investigator: David Kimberlin, MD | Charlotte Hobbs, MD

The Congenital and Perinatal Infections Consortium (CPIC) is focused on reducing the morbidity and mortality of rare viral infections such as congenital cytomegalovirus (CMV) disease, neonatal herpes simplex virus (HSV) infection, and neonatal viral sepsis caused by enteroviruses (EVs) and the related human parechoviruses (HPeVs). These infections have been grouped together because of their pathogenic potential in the neonatal population and the current and future opportunities to intervene meaningfully to improve outcomes. Though antiviral therapeutic agents with activity against each of these viruses already exist (CMV, HSV) or are in development (EV, HPeV), collective consequences include developmental and motor delays, neurologic morbidity, visceral organ damage, hearing and vision loss, respiratory and cardiac complications, septic shock, and death. The opportunity to reduce the impacts of these diseases forms a common purpose among the 29 consortium members. Led by the University of Alabama at Birmingham, these 29 sites have cooperated closely for decades as the Collaborative Antiviral Study Group to investigate the natural history and treatment of rare congenital and perinatal infectious diseases, and will continue to advance their research as an RDCRN consortium.
Consortium Project Manager: Jill Bailey-Griffin, RN, MSN
Contact Information: jillgriffin@uabmc.edu
CPIC is funded under grant number U54AI150225 as a collaboration between NCATS and the National Institute of Allergy and Infectious Diseases (NIAID).
Consortium of Eosinophilic Gastrointestinal Disease Researchers
CEGIR
Consortium of Eosinophilic Gastrointestinal Disease Researchers
Grant number: U54 AI117804-06
Principal Investigator: Marc E. Rothenberg, MD, PhD

Eosinophilic esophagitis, eosinophilic gastritis, eosinophilic gastroenteritis, and eosinophilic colitis are disorders in which a type of immune cell (called eosinophils) builds up in the digestive tract, causing gastrointestinal tissue damage. These disorders are painful, lifelong, and make it hard or impossible for people to eat many or all foods. The Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR) includes clinical centers in Arkansas, California, Colorado, Illinois, Maryland, Massachusetts, Minnesota, New York, North Carolina, Ohio, Pennsylvania, Texas and Utah. CEGIR’s goals are to improve the lives of patients with eosinophilic gastrointestinal diseases through research. CEGIR is conducting innovative clinical studies including interventional trials. CEGIR is collecting clinical information and biological samples from patients to better understand, diagnose and treat these conditions; develop the best methods to measure symptoms and track patients; develop better treatments; make data available to patients, clinicians, researchers, and the public; and engage and cooperate with patient advocacy groups.
Consortium Project Manager: Rachel Andrews | Regina Yearout
Contact Information: rachel.andrews@childrenscolorado.org | regina.yearout@cchmc.org
CEGIR is funded under grant number U54AI117804 as a collaboration between NCATS, the National Institute of Allergy and Infectious Diseases (NIAID), and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
Developmental Synaptopathies Consortium
DSC
Developmental Synaptopathies Consortium
Grant number: U54 NS092090-06
Principal Investigator: Mustafa Sahin, MD, PhD

Autism spectrum disorder (ASD) affects 1 in 59 children in the United States and is a major public health concern and challenge. The Developmental Synaptopathies Consortium (DSC) aims to explore the underlying causes of autism by focusing on three rare genetic disorders related to ASD, including Tuberous Sclerosis Complex (TSC), PTEN Hamartoma Tumor Syndrome (PHTS) and Phelan-McDermid Syndrome (PMS). Understanding the pathophysiology of these rare disorders will lead to the discovery of therapeutic targets, which is the long-term goal of the DSC. The vast data and deep phenotyping of participants with TSC, PTEN and PMS collected to date will be further expanded in this new cycle of the project to include collection of data into adulthood for each disorder. This will allow for additional understanding of the trajectory of each disease and inform not only the research and clinical community, but also other groups that support and educate people affected by these disorders. Additionally, the electrophysiological investigation of these three related diseases will help in the exploration of translational biomarkers and new therapeutic strategies.
Consortium Project Manager: Rajna Filip-Dhima, MS
Contact Information: rajna.filip-dhima@childrens.harvard.edu
DSC is funded under grant number U54NS092090 as a collaboration between NCATS, the National Institute of Neurological Disorders and Stroke (NINDS), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute on Deafness and Other Communication Disorders (NIDCD).
Global Leukodystrophy Initiative Clinical Trials Network
GLIA-CTN
Global Leukodystrophy Initiative Clinical Trials Network
Grant number: U54 NS115052-01
Principal Investigator: Adeline L. Vanderver, MD | S. Ali Fatemi, MD, MBA | Florian S. Eichler, MD

Leukodystrophies are a complex, often progressive group of disorders affecting the white matter of the brain due to the loss or absence of myelin, the lipid membrane that insulates axons in the central nervous system. Despite advances in the diagnosis of these disorders, they remain widely under-recognized, with unmet gaps in clinical care and curative therapeutics. The Global Leukodystrophy Initiative Clinical Trials Network (GLIA-CTN) is a consortium of scientists, industry stakeholders, and patient advocacy leaders working together to promote advances in the diagnosis and treatment of leukodystrophies. Specifically, it seeks to create a robust research infrastructure that will allow for collection and analysis of longitudinal natural history data, development of novel clinical outcome assessments, and identification of surrogate biomarkers, ultimately paving the way for transformative therapeutic trials across the leukodystrophies. In parallel to these approaches, the GLIA-CTN will work closely with a diverse group of stakeholders to promote disease awareness and education—including newborn screening and early diagnosis—and establish clinical guidelines to support the short- and long-term care of individuals living with leukodystrophies.
Consortium Project Manager: Omar Sherbini, MPH
Contact Information: sherbinio@chop.edu
GLIA-CTN is funded under grant number U54NS115052 as a collaboration between NCATS, the National Institute of Neurological Disorders and Stroke (NINDS), and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD).
Initiation of a cohort to define pathogenic Mechanisms, Precision diagnosis And Complications of Thrombotic Microangiopathies: The IMPACT Study
IMPACT
Initiation of a cohort to define pathogenic Mechanisms, Precision diagnosis And Complications of Thrombotic Microangiopathies: The IMPACT Study
Grant number: U54 HL185004-01
Principal Investigator: Robert Brodsky, MD

Consortium Project Manager:
Contact Information:
IMPACT is funded under grant number U54HL185004 as a collaboration between NCATS, and the National Heart, Lung, and Blood Institute (NHLBI).
Myasthenia Gravis Rare Disease Network
MGNet
Myasthenia Gravis Rare Disease Network
Grant number: U54 NS115054-01
Principal Investigator: Henry Kaminski, MD | Richard Nowak, MD | Vern Juel, MD

Myasthenia gravis (MG) is an autoimmune disease that blocks the signal from nerve to muscle, producing weakness. The nature of the disease can range from isolated severe vision problems, like drooping eyelids or double vision, to profound general weakness leading to breathing muscle failure. Although the cause of MG is not known, the disease appears to vary based on several factors, including the types of antibodies injuring the muscle, age, sex, and abnormalities of the thymus gland, which is an important part of the immune system. The Myasthenia Gravis Rare Disease Network (MGNet) serves as a centralized, international consortium for physicians, scientists, and patient advocacy groups as well as industry partners to develop resources and implement ideas to enhance discovery, treatment, and advocacy for the patient community. The immediate goals of MGNet are to further define how patients respond to conventional treatments over time, evaluate a novel drug for therapy of some forms of MG, find biological markers of the disease (biomarkers), and encourage new scientists to focus research on MG.
Consortium Project Manager: Olivia Haddad
Contact Information: ohaddad@mfa.gwu.edu
MGNet is funded under grant number U54NS115054 as a collaboration between NCATS and the National Institute of Neurological Disorders and Stroke (NINDS).
Network for Advancing Sex Chromosome Aneuploidy Research Readiness
NASCARR
Network for Advancing Sex Chromosome Aneuploidy Research Readiness
Grant number: U54 HD121580-01
Principal Investigator: Nicole Tartaglia, MD

The Network for Advancing Sex Chromosome Aneuploidy Research Readiness (NASCARR) is a network of Sex Chromosome Aneuploidy (SCA) researchers and advocacy group partners seeking to advance clinical trial readiness and improve outcomes in SCA.
Consortium Project Manager: Kayla Molison
Contact Information: KAYLA.MOLISON@UCDENVER.EDU
NASCARR is funded under grant number U54HD121580 as a collaboration between NCATS, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Human Genome Research Institute (NHGRI).
North American Mitochondrial Disease Consortium
NAMDC
North American Mitochondrial Disease Consortium
Grant number: U54 NS078059-09
Principal Investigator: Michio Hirano, MD

Mitochondrial diseases affect approximately 1 in every 5,000 people. These diseases can cause muscle weakness, difficulty thinking, seizures, hearing and vision loss, digestive problems, learning disabilities, and organ failure. The North American Mitochondrial Disease Consortium (NAMDC) is a network of clinicians and researchers at 17 different clinical sites working to better understand mitochondrial diseases, improve diagnosis and explore treatments. In the past eight years, NAMDC has established a biorepository for tissue samples, a large registry and follow-up study with more than 1,500 patients, a website to educate and recruit patients, and studies to define the natural history of mitochondrial diseases. NAMDC also has initiated research studies that focus on several subtypes of mitochondrial diseases as well as a training program to educate the next generation of researchers. During the next few years, NAMDC will expand its clinical registry study and biorepository, perform advanced molecular diagnostic testing, conduct natural history and treatment studies, and continue training future clinician-scientists.
Consortium Project Manager: Janet DeRosa, MPH
Contact Information: jtd5@cumc.columbia.edu
NAMDC is funded under grant number U54NS078059 as a collaboration between NCATS, the National Institute of Neurological Disorders and Stroke (NINDS), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the Office of Dietary Supplements (ODS).
Pediatric Fungal Network STudy of Rare Invasive Fungal DisEases in Immunocompromised Pediatric Patients
PFN-STRIDE
Pediatric Fungal Network STudy of Rare Invasive Fungal DisEases in Immunocompromised Pediatric Patients
Grant number: U54 AI198184-01
Principal Investigator: Brian Fisher, DO, MPH, MSCE | William Steinbach, MD

Invasive fungal diseases (IFD) are infections caused by opportunistic fungi that are ubiquitous in the environment and often prey selectively on vulnerable populations, including patients who are immunocompromised. Although rare, IFDs are associated with significant morbidity and short-term case fatality rates of more than 30%. Current diagnostic approaches are extremely limited, often requiring risky invasive procedures, and management is often prolonged and sometimes ineffective. In immunocompromised children, IFDs are rarer and even more complicated to diagnose and manage than those in adults. PFN-STRIDE will leverage the existing Pediatric Fungal Network (PFN), the largest and most productive pediatric consortium dedicated to improving the outcomes of IFDs in pediatric patients. The PFN is currently composed of pediatric academic centers in the United States, each with recognized leaders in pediatric IFD serving as site investigators. Over 35 of these pediatric academic centers have participated in recent R01-funded PFN prospective cohort studies. The overall objectives of PFN-STRIDE are to document long-term outcomes and patient and caregiver perspectives of rare IFD, to better characterize the genetic and susceptibility phenotypes of pathogens causing IFD so that future therapeutic discovery and antifungal clinical trials can be more intentional, and to more fully understand how the host immune system responds to IFD so that novel diagnostic testing approaches can be developed.
Consortium Project Manager: Morgan Hammershaimb
Contact Information: PFNCHOP@chop.edu
PFN-STRIDE is funded under grant number U54AI198184 as a collaboration between NCATS, the National Institute of Allergy and Infectious Diseases (NIAID), and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD).
Phenylalanine Families and Researchers Exploring Evidence
PHEFREE
Phenylalanine Families and Researchers Exploring Evidence
Grant number: U54 HD100982-01
Principal Investigator: Cary Harding, MD

The Phenylalanine Families and Researchers Exploring Evidence (PHEFREE) Consortium studies the health, neurologic, cognitive, neuropsychiatric, patient-reported and quality-of-life outcomes in individuals with chronic elevations of the amino acid phenylalanine in blood (hyperphenylalaninemia). In the United States, elevated blood phenylalanine is typically detected at birth through newborn screening and is caused by inherited disorders affecting the metabolism of phenylalanine. The most common disorder is phenylketonuria (PKU), but the consortium also studies other rare disorders of phenylalanine metabolism, including defects in biopterin synthesis or recycling, or deficiency of the chaperone protein DNAJC12. In partnership with a patient advocacy group, the National PKU Alliance (NPKUA), the consortium provides informational and educational resources to patients, their families, their providers and the public regarding these rare disorders and trains the next generation of rare disease researchers and practitioners. The consortium has formed a clinical trial network with the facile ability to evaluate emerging novel assessment methods or therapies for these diseases. Its goals are to assess the longitudinal outcomes of this patient population and refine and improve current and future therapies for these disorders in phenylalanine metabolism.
Consortium Project Manager: Hadley Morotti, MS
Contact Information: phefree@ohsu.edu
PHEFREE is funded under grant number U54HD100982 as a collaboration between NCATS, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Institute of Neurological Disorders and Stroke (NINDS), and the Office of Dietary Supplements (ODS).
Rare Bronchiectatic Diseases Consortium
RBDC
Rare Bronchiectatic Diseases Consortium
Grant number: U54 HL185005-01
Principal Investigator: Kenneth Olivier, MD, MPH

The Rare Bronchiectatic Diseases Consortium (RBDC) studies four rare diseases: primary ciliary dyskinesia, alpha1 antitrypsin deficiency, humoral immunodeficiencies, and STAT3hyper IgE syndrome. These four rare diseases share bronchiectasis as a serious and progressive pulmonary complication marked by chronic infection and airway inflammation. Therapeutic development for bronchiectasis has historically lagged, in part due to limited understanding of disease trajectories and the biologic mechanisms that initiate and perpetuate airway injury. In partnership with Patient Advocacy Groups and the Rare Diseases Clinical Research Network, the consortium aims to advance awareness of these underrecognized conditions, define their longitudinal clinical course, and generate high quality clinical and biomarker data essential for future clinical trials. Guided by new insights from our updated “Vicious Vortex 2.0” model of bronchiectasis pathogenesis, built off of the Vicious Vortex model proposed by Flume1, the consortium seeks to identify novel therapeutic targets and treatable traits. Through these efforts, we aim to accelerate drug discovery, strengthen the pipeline of rare disease investigators, and ultimately improve outcomes for individuals affected by these conditions.
References
1. Flume PA, Chalmers JD, Olivier KN. Advances in bronchiectasis: endotyping, genetics, microbiome, and disease heterogeneity. Lancet. 2018;392(10150):880-90. doi: 10.1016/S0140-6736(18)31767-7. PubMed PMID: 30215383; PMCID: PMC6173801.
Consortium Project Manager: Gabriella Keller | Joseph Hatch, MSPH | Kelli M. Sullivan, MPH
Contact Information: gabriella_keller@med.unc.edu | hatchjo@email.unc.edu | kelli_sullivan@med.unc.edu
RBDC is funded under grant number U54HL185005 as a collaboration between NCATS and the National Heart, Lung, and Blood Institute (NHLBI).
Rare Organic Acidemias Research Consortium
ROAR
Rare Organic Acidemias Research Consortium
Grant number: U54 HD121579-01
Principal Investigator: Vernon Sutton

Consortium Project Manager:
Contact Information:
ROAR is funded under grant number U54HD121579 as a collaboration between NCATS, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Human Genome Research Institute (NHGRI).
Spastic Paraplegia Centers of Excellence Research Network
SP-CERN
Spastic Paraplegia Centers of Excellence Research Network
Grant number: U54 NS148312-01
Principal Investigator: Darius Ebrahimi-Fakhari, MD, PhD

The Spastic Paraplegia Centers of Excellence Research Network (SP-CERN) - RDCRC brings together 11 clinical and research centers across North America, led by physician-scientists and multidisciplinary teams. SP-CERN coordinates collaborative research efforts to advance the understanding, diagnosis, and treatment of hereditary spastic paraplegia (HSP), establishing a national infrastructure to support natural history studies, biomarker development, and clinical trial readiness.
Consortium Project Manager:
Contact Information:
SP-CERN is funded under grant number U54NS148312 as a collaboration between NCATS, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Human Genome Research Institute (NHGRI), and the National Institute of Neurological Disorders and Stroke (NINDS).
Urea Cycle Disorders Consortium
UCDC
Urea Cycle Disorders Consortium
Grant number: U54 HD061221-16
Principal Investigator: Andrea Gropman, MD

Urea cycle disorders (UCDs) are rare but devastating genetic conditions. In 2003, the Urea Cycle Disorders Consortium (UCDC) became one of the first members of the RDCRN. Since then, UCDC has flourished into an international network of 16 academic centers in the United States, Canada, and Europe that provide state-of-the-art care and conduct cutting-edge clinical research. The UCDC is currently focusing on research on three major studies as well as pilot studies on innovative diagnostics and therapies.
The three major protocols include a long-term natural history study of patients with UCDs that has been active since 2006; a project investigating sub-clinical seizures in neonates with hyperammonemia and its impact on the development of epilepsy, neurodevelopment and affect brain function and biochemistry; and an MRI study of liver fibrosis as a complication of UCDs. The consortium also continues to train the next generation of rare diseases researchers, provide education on UCDs to health care professionals and the public in partnership with the National Urea Cycle Disorders Foundation, and work with the pharmaceutical/biotechnology industry to develop and test novel medications and treatments to help improve the lives of those affected by UCDs.
Consortium Project Manager: Jennifer Seminara, MPH, CCRP
Contact Information: jseminar@childrensnational.org
UCDC is funded under grant number U54HD061221 as a collaboration between NCATS, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
