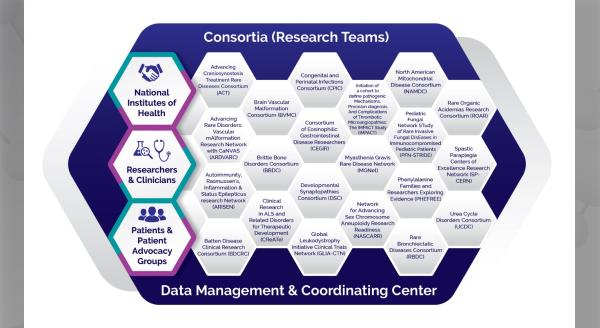
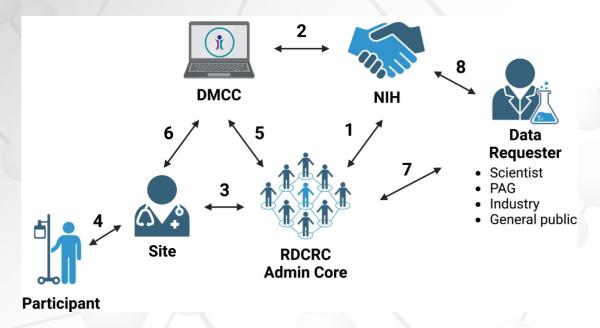
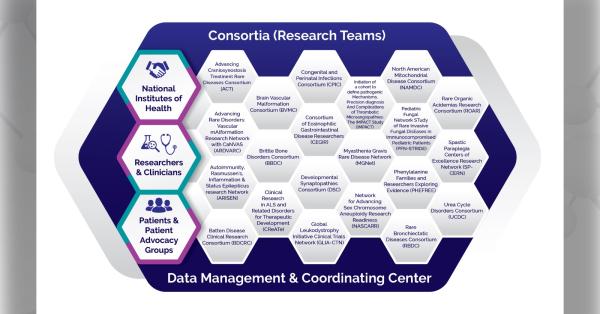
The Congenital and Perinatal Infections Consortium (CPIC) is a group of scientists, clinicians, stakeholders, and patient advocates focused on rare congenital and perinatal viral infections. These infections include congenital cytomegalovirus(CMV) disease, neonatal herpes simplex virus (HSV) infection, and neonatal viral sepsis caused by enteroviruses (EVs) and the related human parechoviruses (HPeVs). Here, principal investigator David Kimberlin, MD, and pilot program administrator Cheryl Perry, MPH, PhD, share the history of the consortium, current research, and future plans.
What are the overall goals of the CPIC?
We aim to advance the health of children afflicted with life-threatening viral infections acquired before or during delivery. The CPIC is working toward this goal by establishing an infrastructure and facilitating the institutional cooperation necessary to advance understanding of these diseases, train future researchers, improve clinical trial readiness, test therapies, advance patient care, and ultimately reduce disease burden.
How did the CPIC team come together, and how did you become a part of the RDCRN?
Our sites have existed as the CPIC since 2019. Prior to this, most worked together for over two decades as part of the NIH-funded Collaborative Antiviral Study Group (CASG) to investigate the natural history and treatment of rare congenital and perinatal infectious diseases. These longstanding relationships have guided development of our research portfolio and greatly contributed to and accelerated the success of our overall program. Together, the CPIC sites continue to facilitate clinical trial readiness and provide mentored research opportunities to train future rare diseases researchers.
Can you tell us more about the rare diseases you study?
A congenital or perinatal infection is an infection caused by a bacteria or virus that can be passed from a mother to her baby during pregnancy (congenital) or delivery (perinatal).
CPIC evaluates:
- Congenital cytomegalovirus (CMV) disease. About one in 200 infants in the US are born with CMV disease, and of those, about 20% will develop symptoms as they grow, including serious complications like hearing loss, visual impairment, intellectual disability, or epilepsy.
- Neonatal herpes simplex virus (HSV) infection. Disseminated disease is the most serious manifestation of neonatal HSV and has the highest mortality, while central nervous system (CNS) disease leads to significant lifelong morbidity in about a third of survivors. Infected neonates generally become ill at 2-3 weeks of life.
- Neonatal sepsis caused by EVs or HPeVs. This is a viral infection that can lead to CNS and cardiovascular complications, liver damage, and death when acquired around the time of delivery. Infected neonates generally become ill within the first 10 days or so of life.
Though antiviral therapeutic agents already exist (CMV, HSV) or are in varying stages of clinical development (EV, HPeV), neonates and young infants still experience unacceptable adverse consequences of these diseases, including developmental and motor delays, neurologic morbidity, visceral organ damage, hearing and vision loss, respiratory and cardiac complications, septic shock, and death.
What are your current research projects?
- The Neonatal EV Sepsis protocol is defining the morbidity and mortality of neonatal EV and HPeV sepsis to identify endpoints for subsequent studies of antiviral drugs that are in development. Neonates with clinical signs and laboratory data consistent with viral sepsis (without a diagnosis of bacterial, HSV, CMV, or adenovirus sepsis) are included in this prospective natural history study. Babies are followed for three months to determine morbidity and mortality associated with their disease, and to explore quantitative changes in the virus over time. During the study, babies receive all clinical interventions and management deemed necessary by their treating physicians. This study is open and enrolling at 29 sites.
- The Phase I Valacyclovir PK study in neonates potentially exposed to HSV at delivery will provide critical data to determine the optimal valacyclovir dose and describe the safety profile of valacyclovir therapy for a future, larger efficacy study. Enrolled neonates will receive a short course of oral valacyclovir, with pharmacokinetic assessments obtained to determine the best dose for this age group. These data will be analyzed along with existing datasets in slightly older infants to model the ideal drug exposures needed. Neonates whose mothers have a history of genital HSV infection and are taking oral acyclovir or valacyclovir for several weeks prior to delivery, as per recommendations of the American College of Obstetricians and Gynecologists, will be enrolled. The study start-up meeting was held on August 24, 2022.
- Our Retrospective Follow-up CMV study evaluates children who were born with symptomatic congenital CMV disease and who previously received valganciclovir and/or ganciclovir therapy to determine durability of the beneficial treatment effects on hearing and developmental outcomes. Subjects will have their hearing and development re-evaluated during a one-time visit, along with assessment for possible long-term toxicities of therapy. The study start-up meeting was held on August 30, 2022.
- The Phase I Letermovir PK study will advance the treatment of babies with symptomatic congenital CMV by defining the pharmacokinetics and safety of letermovir, the first new CMV antiviral to be approved by the US Food and Drug Administration in over two decades. This study is scheduled to begin toward the end of the year.
How has your research impacted patients?
Our CPIC studies are in relatively early stages—either currently enrolling or soon to be initiated. However, the CPIC’s predecessor, the CASG, has conducted large, multicenter, NIH-funded studies of rare diseases since the 1970s. Data generated by the CASG have led to nine label (Package Insert) changes for rare viral diseases affecting newborns and young children, and have established the standard of care for treatment of four diseases. These include treatment of congenital CMV disease, neonatal HSV infection, infantile influenza, and varicella-zoster virus infections.
What new research directions or goals are you pursuing?
Two additional areas of focus that we are undertaking include infantile parechovirus central nervous system infections and enterovirus D68 respiratory infections. For the former, a number of CPIC sites will contribute to a large case series that is being developed by one of our CPIC site investigators. For the latter, we will participate in a study run through the NIH Vaccine Research Center that seeks to follow patients over the course of a year for acquisition of this emerging infection to answer immunologic questions that could inform future studies of other emerging infections.
How has being part of the RDCRN impacted the CPIC’s work?
Being part of the RDCRN has facilitated interaction with other groups who have a similar focus, such as the partnership we have developed with the Primary Immune Deficiency Treatment Consortium (PIDTC) to run CMV virology samples for their study of CMV in infants with severe combined immunodeficiency disease (SCID). It has also allowed us to share our expertise in trial design, site activation, and pharmacokinetics/pharmacodynamics (PK/PD) with other consortia, such as Phenylalanine Families and Researchers Exploring Evidence (PHEFREE).
In addition, it has facilitated seamless incorporation of another patient advisory group (PAG), Herpes Cure Advocacy, which is relying on other RDCRN PAGs as mentors. CPIC studies have served as test cases for Single IRB support of RDCRN studies by the DMCC, and the development of registries for rare diseases.
How do you envision that the diseases you study can ultimately be cured?
Many of the diseases that the CPIC studies are herpesviruses, which by definition establish latency in the body following initial infection and thus cannot be “cured” in the traditional sense. That said, our studies can improve outcomes by setting the stage for combination therapy (congenital CMV), initiating preemptive therapy to prevent transmission (neonatal HSV), and defining outcomes for future studies of antiviral drugs in development (neonatal enteroviral disease).
The Congenital and Perinatal Infections Consortium (CPIC) is part of the Rare Diseases Clinical Research Network (RDCRN), which is funded by the National Institutes of Health (NIH) and led by the National Center for Advancing Translational Sciences (NCATS) through its Division of Rare Diseases Research Innovation (DRDRI). CPIC is funded under grant number U54AI150225 as a collaboration between NCATS and the National Institute of Allergy and Infectious Diseases (NIAID).