For over 20 years, the Rare Diseases Clinical Research Network (RDCRN) has been working to discover the best ways to achieve faster diagnoses and better treatments in rare diseases. While promising treatments are in development, it is important to understand the natural history of the diseases, including the signs of increased disease severity and disability that may become the target of new treatments.
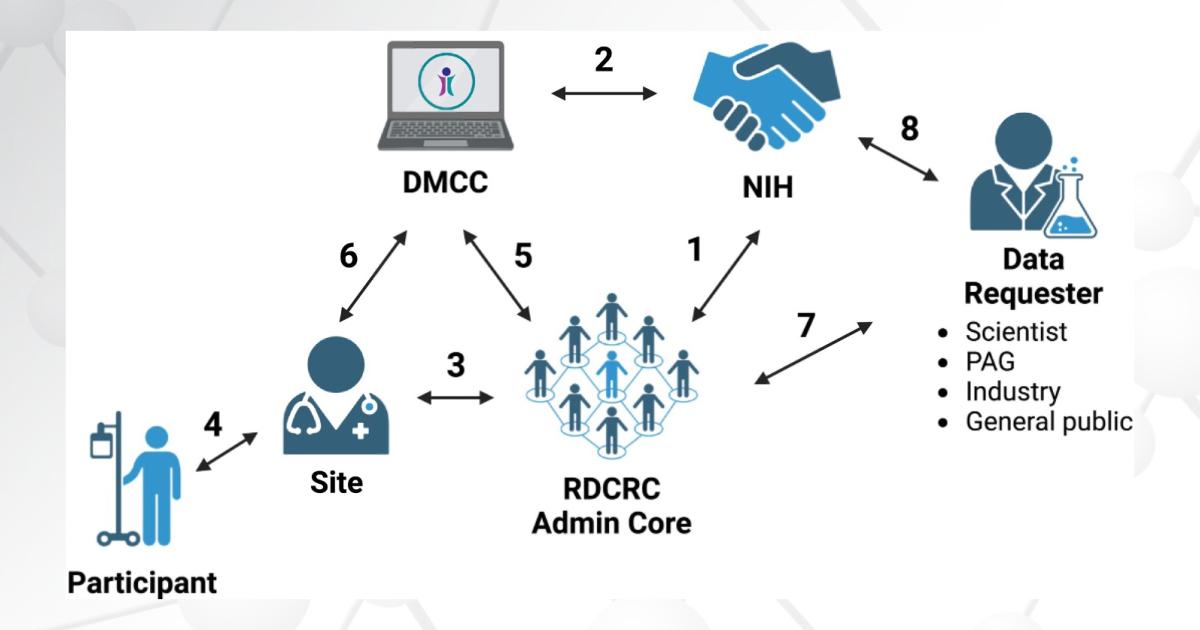
Many RDCRN studies do just that, and the data collected by the RDCRN today offers valuable information for academic researchers and industry to plan the studies of tomorrow. Thus, it is critically important for the RDCRN to have principles and guidance to ensure that the research teams comprising the network can share data with others.
In a new publication, “Data Sharing Experience, Guidance, and Resources From the Rare Diseases Clinical Research Network (RDCRN),” lead author Elaine Schwendeman, PhD, PMP, and senior author Maurizio Macaluso, MD, DrPH, FACE, highlight this forward-looking aspect of the RDCRN research.
What is data sharing and how does it impact rare disease research?
Dr. Schwendeman: Data sharing is a term used to describe the practice of releasing research study data to other researchers for future scientific study, directly or through a data repository, and at the same time respecting the privacy of a research participant.
A rare disease, by definition, is a condition that affects fewer than 200,000 people in the United States. Due to the limited number of people affected by any singular rare disease, it is challenging to enroll sufficient numbers of participants in research studies; data sharing is critical to advance knowledge in the field.
Why is it important to develop and follow best practices for data sharing?
Dr. Macaluso: Data sharing policies don’t need to be identical among researchers and institutions, but to be successful, common goals and practices are necessary. The 2023 Final NIH Policy for Data Management and Sharing sets the expectation that researchers will maximize the appropriate sharing of scientific data, acknowledging certain limitations, and that scientific data should be made available as soon as possible.
The RDCRN Data Management and Coordinating Center (DMCC), in collaboration with the RDCRN Data Use and Data Sharing Committee, assembled data sharing guidance for the network, in line with the 2023 NIH Policy, to promote common practices and enhance data sharing across the network.
What are some key points to know about data sharing?
Dr. Schwendeman: Data sharing begins with the research participant, who must grant their approval through an informed consent process. This process educates the participant on how their data will be used and shared and how their confidentiality will be protected.
It is critical to protect the privacy and confidentiality of research participants, which is especially true in the study of rare diseases, where characteristics of the disease or condition may make it easier to identify someone in a research study. Not all research data can be shared.
The researcher and institutional ethics committee must make decisions about what data is appropriate to share while protecting a participant’s privacy. Research data sharing, for studies funded by the National Institutes of Health (NIH), is subject to the 2023 Final NIH Policy for Data Management and Sharing, among other policies.
Which RDCRN groups collaborated to develop the guidance?
Dr. Macaluso: The RDCRN Data Use and Data Sharing Committee, in collaboration with the RDCRN DMCC, reviewed the data sharing policies and practices of research consortia within the network.
By identifying common successful data sharing practices among RDCRN consortia and following the principles outlined in the 2023 Final NIH Policy for Data Management and Sharing, the Committee and the DMCC developed the RDCRN Suggestions for Establishing Data Sharing and Data Management Guidance for Individual Consortia. This guidance promotes common practices within the network, facilitating research data sharing.
How can effective data sharing impact the rare disease community?
Dr. Schwendeman: RDCRN data is precious due to the rarity of the conditions studied and multi-site collaborations are necessary to enroll sufficient numbers of participants, making data sharing among sites critical for a study to succeed.
According to the PAR-24-206 and PAR-25-438 Notice of Funding Opportunities for the RDCRN, advancing rare disease research by freely sharing high-value data is a critical goal of the program. Deidentified data collected within the network will become a resource for the greater rare disease research community.
Effective data sharing facilitates future research and can lead to a greater understanding of rare diseases, which may help speed advances in the field.
Read the full publication in the journal Clinical and Translational Science.