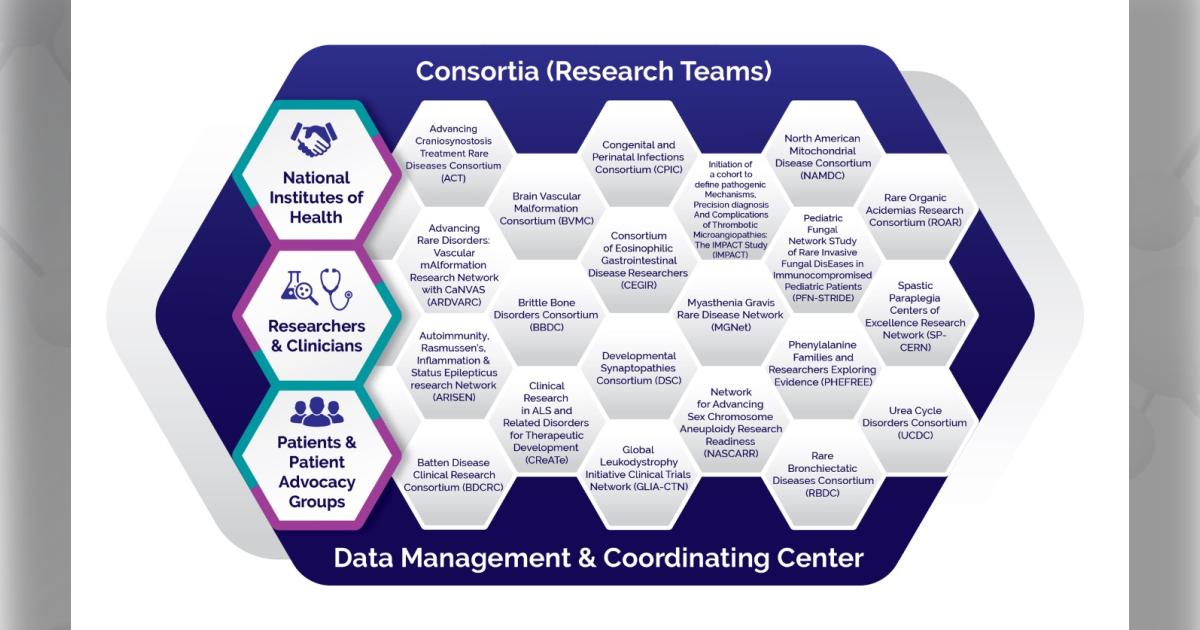
The National Institutes of Health (NIH) has awarded approximately $26 million in grants in the fiscal year 2025 to begin the fifth cycle of funding for the Rare Diseases Clinical Research Network (RDCRN). This national network brings together scientists, clinicians, patients, families, and patient advocates to study a wide range of rare diseases.
Today, the network consists of 21 research consortia, including 10 that are joining the network for the first time, five that were active in the previous cycle and have been awarded a five-year renewal, and six Cycle 4 consortia that are continuing through a one-year extension of their previous award. An additional $5.6 million has been awarded to a separate Data Management and Coordinating Center hosted at Cincinnati Children’s Hospital Medical Center to support these research efforts.
Addressing the Challenges of Rare Diseases
About one in every nine Americans is affected by a rare disease. Of the approximately 10,000 known rare diseases, only around five percent have US Food and Drug Administration (FDA)-approved treatments. To get a drug from discovery to market, it can take as long as 10 to 15 years and cost up to $2.6 billion. And because most rare diseases are genetic, they often affect infants and children.
To advance rare disease research, Congress passed a law in 2002 authorizing several agencies within the NIH to launch the RDCRN. Each of the 21 consortia that make up the network study at least three different rare diseases.
Since its establishment, the RDCRN has supported hundreds of studies at over 300 clinical sites around the world. These efforts have resulted in over 3,000 publications with topics ranging from natural history studies and case reports to practice guidelines and clinical trials of new treatments. Findings from these studies have contributed to the approval of 11 treatments for rare diseases by the FDA.
Expanding the Focus to New Diseases
The new consortia include:
- Advancing Craniosynostosis Treatment (ACT) Rare Diseases Consortium
- Lead institution: Children's Hospital of Philadelphia
- Advancing Rare Disorders: Vascular mAlformation Research Network with CaNVAS (ARDVARC)
- Lead institution: Children's Hospital of Philadelphia
- Autoimmunity, Rasmussen’s, Inflammation & Status Epilepticus research Network (ARISEN)
- Lead institution: Emory School of Medicine
- Batten Disease Clinical Research Consortium (BDCRC)
- Lead institution: Kennedy Krieger Institute
- Initiation of a cohort to define pathogenic Mechanisms, Precision diagnosis And Complications of Thrombotic Microangiopathies: The IMPACT Study (IMPACT)
- Lead institution: Johns Hopkins Medicine
- Network for Advancing Sex Chromosome Aneuploidy Research Readiness (NASCARR)
- Lead institution: Children's Hospital Colorado Anschutz Medical Campus
- Pediatric Fungal Network STudy of Rare Invasive Fungal DisEases in Immunocompromised Pediatric Patients (PFN-STRIDE)
- Lead institution: Children's Hospital of Philadelphia
- Rare Bronchiectatic Diseases Consortium (RBDC)
- Lead institution: University of North Carolina at Chapel Hill
- Rare Organic Acidemias Research Consortium (ROAR)
- Lead institution: Baylor College of Medicine
- Spastic Paraplegia Centers of Excellence Research Network (SP-CERN)
- Lead institution: Boston Children's Hospital
Supporting New Opportunities for Continuing Groups
The consortia returning for Cycle 5 include:
- Brittle Bone Disorders Consortium (BBDC)
- Lead institution: Baylor College of Medicine
- Clinical Research in ALS and Related Disorders for Therapeutic Development (CReATe) Consortium
- Lead institution: University of Miami
- Developmental Synaptopathies Consortium (DSC)
- Lead institution: Boston Children's Hospital
- Global Leukodystrophy Initiative Clinical Trials Network (GLIA-CTN)
- Lead institution: Children's Hospital of Philadelphia
- Myasthenia Gravis Rare Disease Network (MGNet)
- Lead institution: George Washington University
The consortia receiving a no-cost extension for Cycle 4 include:
- Brain Vascular Malformation Consortium (BVMC)
- Lead institution: University of California, San Francisco
- Congenital and Perinatal Infections Consortium (CPIC)
- Lead institution: University of Alabama at Birmingham
- Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR)
- Lead institution: Cincinnati Children's Hospital Medical Center
- North American Mitochondrial Disease Consortium (NAMDC)
- Lead institution: Columbia University Medical Center
- Phenylalanine Families and Researchers Exploring Evidence (PHEFREE) Consortium
- Lead institution: Oregon Health & Science University
- Urea Cycle Disorders Consortium (UCDC)
- Lead institution: Children's National Medical Center
Learn more about the RDCRN and the 21 consortia.