Douglas Marchuk, PhD, is the director of the Division of Human Genetics at Duke University and member of the Brain Vascular Malformation Consortium (BVMC). His laboratory studies the genetics of cardiovascular disease using both the human and the mouse as a model system, primarily focusing on inherited diseases of vascular dysplasia. Here, he shares his start in rare disease research, exciting discoveries, and future goals.
What inspired you to become a researcher in the rare disease space?
I did my postdoctoral fellowship at the University of Michigan with Dr. Francis Collins, who is now the NIH Director. His lab focused on gene discovery for single gene (Mendelian) diseases, which falls within the rare disease space. I worked on neurofibromatosis (NF), a genetic disorder of the nervous system, and was part of the team that discovered the NF gene.
When I left Dr. Collins’ lab, his “farewell gift” to me was a box of DNA tubes from two large families with another inherited disease—hereditary hemorrhagic telangiectasia (HHT). DNA from one family was collected by Dr. Collins and Dr. Michael Iannuzzi, one of his colleagues at the University of Michigan. DNA from the other family—arguably the largest HHT family ever ascertained—was collected by Dr. Alan Guttmacher, who was then at the University of Vermont Medical School.
These tubes of DNA were sitting in the freezer in Dr. Collins’ lab, but no one had begun to use them to find the causative gene. He gave me the box and said, “Go for it!”
With the DNA from these two families, I started my independent career. The HHT Foundation (now Cure HHT) held yearly family conferences and we attended every meeting for over a decade, where we interviewed and drew blood from patients and families. These additional samples formed the basis for our studies to find the three HHT genes.
What has been your biggest “aha” moment as a scientist?
The most significant moment was the very first mutation that we discovered in the endoglin gene in one of our HHT families. It was 1994, my first year at Duke University when I was still building my research team. We found a very clear mutation, and that very day, we knew we had the right gene—even though in the following days and weeks we identified a few other mutations that validated our discovery.
I gathered the whole lab around the autoradiograph of the sequencing gel—now obsolete technology—that identified the first HHT mutation. I said something like this: “The moments when you make a major discovery in your career are going to be rare. You may only have one or two, or at most a handful, in your entire career. THIS is one of those moments.”
And then I said something a bit crazy: “If right now you aren’t so excited that you want to run down the hallway screaming for joy, then you should leave science and find another career, because it won’t EVER get any better than this!” I did honestly say that, and I still believe it is true.
People in my lab have been lucky because we have experienced four or five other major moments of disease-gene discoveries. But for me, the first one identified in my own lab is still the most significant.
Tell us about a recent discovery and what it means for patients and physicians.
As part of the BVMC, my lab was on the team (with Dr. Jonathan Pevsner, his graduate student Matthew Shirley, and pediatric neurologist Dr. Anne Comi of Johns Hopkins University) that identified the somatic mutation that causes Sturge-Weber syndrome (SWS).
This discovery—another “aha” moment—was the beginning of a flurry of research on how this mutation leads to SWS. For patients, this means that we are no longer in the dark regarding the cause of the disease, and we finally have hope that a therapy can be developed to counteract the effects of the mutation.
Tell us about the BVMC, how it came together, and what role it has played in your work.
In 2009, the late Dr. William Young learned of an NIH funding opportunity to create a large consortium to study rare diseases under the Division of Rare Diseases Research Innovation. We had worked together on other projects, but not on the three inherited diseases that I was studying, which are now studied by our BVMC. We chose these diseases because we had already identified the genes for two of them—cerebral cavernous malformations (CCMs) and HHT, so we had a head start.
In parallel and independently, the leaders of the patient advocacy groups for SWS (Karen Ball), HHT (Marianne Clancy), and CCMs (Connie Lee) were discussing this funding opportunity from the perspective of patient advocacy.
Along with other outstanding colleagues, we began working together to build a team for our own consortium. We chose the name because of our focus on rare diseases that are characterized by brain vascular malformations. Dr. Young even designed the logo which we still use today. In 2009, we submitted the NIH grant that first funded the BVMC.
Sadly, Dr. Young passed away late in the first cycle of the BVMC, but his legacy remains with us. He was our leader and one of my personal heroes. If it wasn’t for Dr. William Young, there would be no BVMC.
What do you see ahead for the BVMC and your rare disease research?
The BVMC has provided a foundation for a large team of physicians and scientists to pursue new ideas for the three diseases we study. With the infrastructure, patient registries, talents, and expertise of the collaborative team, physicians and scientists can now test their new ideas quickly—without having to build everything from scratch.
This was again Bill’s genius. He always thought of the BVMC as merely the start of what we could do together if we had a firm foundation. BVMC wasn’t the endgame, it was just the beginning. It was our foundation.
The BVMC as an NIH-funded consortium will cease at the end of the current funding cycle (July 31, 2024). This is because the NIH is “sunsetting” any consortia that will have been funded for three five-year periods—a list of consortia that includes us. We have already begun plans to continue to work together as a consortium, but whether there are other opportunities to fund the full consortium with all three diseases together remains to be seen.
However, we are particularly encouraged that each of the disease-specific research teams remain very committed to collaborating and working together. My own personal goal is to see all three groups continue on in some form—each with the goal of an effective treatment for their respective disease.
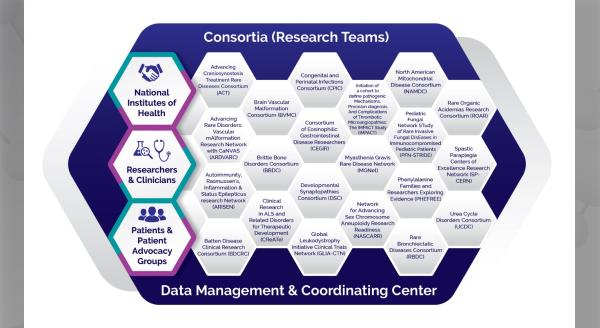
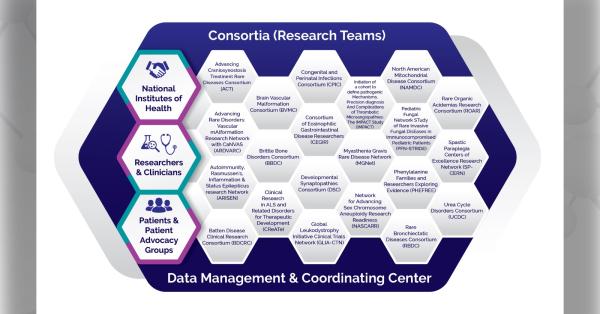
The Brain Vascular Malformation Consortium (BVMC) is part of the Rare Diseases Clinical Research Network (RDCRN), which is funded by the National Institutes of Health (NIH) and led by the National Center for Advancing Translational Sciences (NCATS) through its Division of Rare Diseases Research Innovation (DRDRI). BVMC is funded under grant number U54NS065705 as a collaboration between NCATS and the National Institute of Neurological Disorders and Stroke (NINDS).