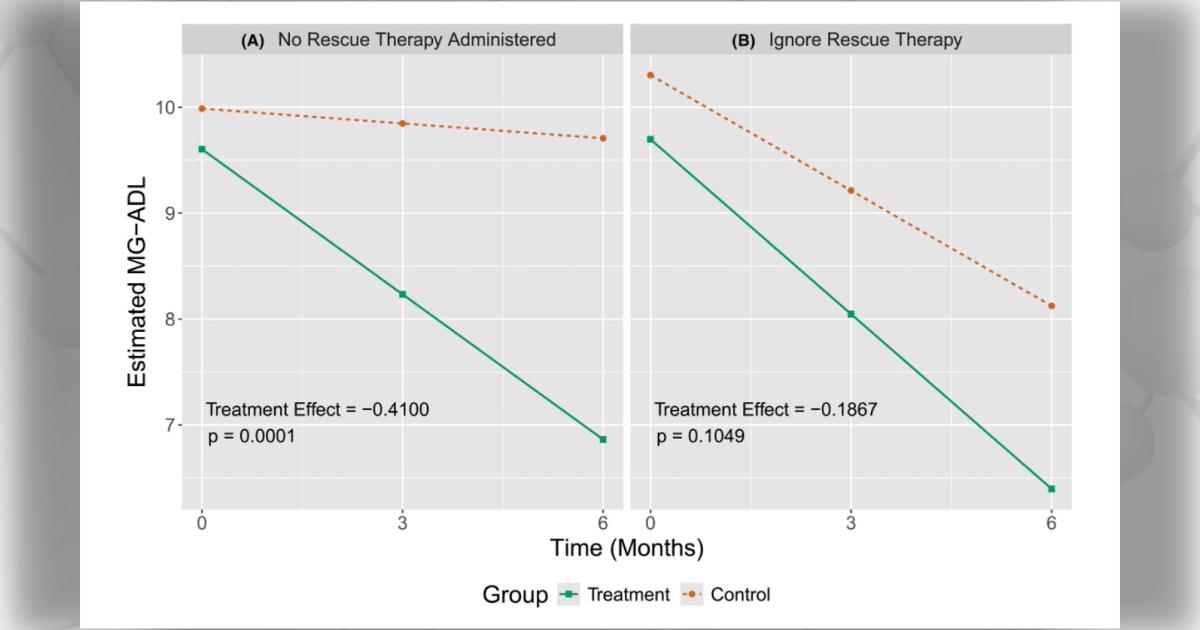
Clinical trials for myasthenia gravis (MG), a chronic autoimmune neuromuscular disorder, aim to evaluate whether a new therapy can improve patients’ symptoms. But what happens if a study participant’s symptoms don’t improve—or even get worse?
Rescue therapy is an intervention that can help improve a study participant’s condition using existing therapies. However, when participants receive rescue therapy before the end of the study, their reaction to the study treatment may change. This can lead to inaccurate results if not appropriately accounted for by study teams.
Statistical models can help, says Justin Leach, PhD, an assistant professor in biostatistics at the University of Alabama at Birmingham and author of a recent pilot study funded by the Myasthenia Gravis Rare Disease Network (MGNet).
“The results of clinical trials influence patient care as well as other scientific and medical research,” says Dr. Leach. “The quality of information arising from such research is dependent in part on robust study design and statistical analysis. Ensuring that we develop and use the most robust statistical methods to address rescue therapy can increase the likelihood of identifying therapies that will benefit patients.”
Dr. Leach and colleagues started by reviewing currently available strategies for addressing rescue therapy in MG trials. They identified five different approaches—treatment policy, hypothetical, while-on-treatment, composite, and principal stratum.
“Each of these approaches can be implemented using a variety of statistical methods,” says Dr. Leach. “Ultimately, the approach taken should depend on the context of the trial, rather than there being a single ‘best’ answer that always applies.”
To help study teams choose the most effective strategy for their trial, Dr. Leach and colleagues outline how each strategy can affect the interpretation of trial results. They also suggest circumstances when each strategy may or may not be applicable to patients or physicians. Considering these guidelines early in the planning process will not only improve the design of clinical trials, but also improve patient care.
“I think rescue therapy will remain necessary in MG trials, especially for trials that focus on patients with higher disease burdens entering the study, in which case rescue therapy rates are likely to be higher,” says Dr. Leach. “This means that it will still be important to appropriately address rescue therapy in statistical analysis plans for MG trials.”
Next, the team plans to conduct statistical simulation studies to better understand how approaches for handling rescue therapy perform in different scenarios.
“We hope that this work will provide additional clarity on the strengths and limitations of these methods that can inform recommendations for circumstances when they are or are not appropriate,” says Dr. Leach. “As a statistician, this is where my work usually comes from and what I am interested in—physicians or other medical professionals with real-world problems that require, at least in part, a statistical solution.”
Read the full study in the journal Annals of Clinical and Translational Neurology.