Myasthenia gravis (MG) is a rare neuromuscular disorder caused by an autoimmune response which blocks or damages acetylcholine receptors on muscles. Most patients with MG experience generalized muscle weakness and fatigue with prolonged activity, which can improve with rest.
To learn more about the use of telehealth in MG, the Myasthenia Gravis Rare Disease Network (MGNet) is conducting a pilot study, “Adapting Disease Specific Outcome Measures Pilot Trial for Telehealth in Myasthenia Gravis (ADAPT-teleMG)” (clinicaltrials.gov ID NCT05917184). The team is evaluating telehealth visits and remote disease-specific assessments for patients with MG.
Here, lead investigator Amanda Guidon, MD, MPH, and Meridith O’Connor, MG patient and assistant vice president of patient engagement, advocacy, and policy at the Myasthenia Gravis Foundation of America (MGFA), share more about the study and its impact on the patient and research community.
Why was it important for patients to join the ADAPT-teleMG study?
O’Connor: Clinical trials in general are more than just a stepping stone to improving the lives of those who are chronically ill; they offer hope when it seems like there is none and opportunities for patients to effect change not just for themselves but for all of those living with a debilitating diagnosis. Participating in a trial such as the ADAPT-teleMG study is a noninvasive way for patients to contribute to the betterment of the MG community and play a critical role in creating equitable access to healthcare through the use of telemedicine.
How was this study designed and performed?
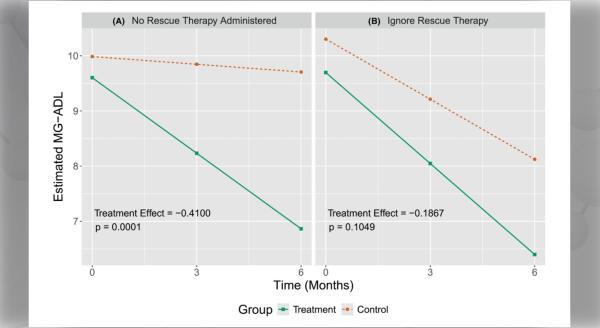
Dr. Guidon: This study was designed to make an urgent pivot from EXPLORE-MG2, MGNet’s natural history study, by necessity during the COVID-19 pandemic. We needed methods to evaluate our patients over telemedicine in a reliable fashion for both research and clinical care. We quickly realized we had no validated tools. The aim was to be able to continue the EXPLORE-MG2 study, but also has ongoing relevance and applications. The primary outcome was the inter and intra-rater reliability of the MG composite virtual (MGCv), a telemedicine-adapted version of the previously validated and commonly used MG composite scale, between the first and second visits.
We enrolled 52 established and stable patients with autoimmune MG from five MGNet sites, completing two telemedicine visits as per study protocol. Multiple patient-reported and physician-reported outcome measures of quality of life, perception of disease severity, fatigue, and weakness were performed.
A portion of the visit—the MG Activities of Living Score (MG-ADL) and the two objective scales derived from the virtual physical examination—was recorded. The video was then sent securely to two independent raters who were also MG experts and who were blinded to other clinical information.
Inter and intra-rater reliability were determined. For disagreement above a pre-determined threshold in any of the three primary scales, the video was sent to a third independent rater. Rating concordance between the site investigator performing the scoring live and the independent raters performing the scoring from the recorded videos was assessed.
What makes this study unique?
Dr. Guidon: To our knowledge, this study is the first study in MG which was performed entirely virtually, all the way from consent to clinical examination assessments. It was also the first to compare clinical exams made in real time to ratings of the same exams done “asynchronously” from recorded videos by investigators who were blinded to the clinical information of the participants.
What excites you about this study?
O’Connor: Technology is changing the future of healthcare, and I am thrilled that researchers are not only recognizing the impact this could have on those living with MG but are inviting MG community members to participate because they see value in their patient experiences.
What are we learning about MG from this study?
Dr. Guidon: In addition to the data generated by the study and possibly some new tools, we have learned a lot about the way that we appreciate and describe the spectrum of phenotypes of MG. I think the more we deconstruct patterns of weakness and abnormal movement, including through the virtual lens, the more we understand disease. This can only be a positive step forward in developing treatments which are effective in treating the most disabling aspects of MG.
The study also brought to light some of the challenges of standardization of outcome measures, particularly in a fluctuating disease such as MG. These lessons learned will inform both virtual and in-person outcome measurement development in the future.
How will this study contribute to clinical trial readiness?
Dr. Guidon: Preliminary data regarding the primary outcome shows excellent intra and inter-rater reliability of both the MGCv and the MG-CE. This opens possibilities for centralized raters in clinical trials in MG. This has potential benefits for increased rating standardization as well as the possibility for substitution of some in-person visits with telemedicine. This could reduce the overall cost of conducting trials. Patients may be more inclined to participate in clinical research, particularly observational natural history studies, if traveling to the site for every visit is not required.
We are beginning to see the data from the perceptions of the utility of telemedicine and satisfaction with the assessments. We see that while satisfaction with the scales and the Zoom platform was generally good, there was variability, and multiple participants—both investigators and patients—cited the need for development of better technology.
How will this study impact patients?
Dr. Guidon: This study provides validated clinical research tools and knowledge of disease natural history necessary for the design of efficient clinical trials. This study also provides sufficient data that the MGCv should be investigated further as a remote outcome measure in virtual clinical care and clinical trials. If responsive to change and generalizable, we could potentially have hub and spoke clinical and research networks to reach a population more representative of the actual real-world population of MG patients and to also reach areas nationally and globally without specialized MG care.
How could this study impact your life?
O’Connor: As an MG patient, I’m looking forward to seeing where this will lead and know it will only enhance the opportunity for patients to have options in how they receive healthcare.
What is the status of this study, and what’s next?
Dr. Guidon: The study has completed enrollment and final data analysis is underway, including all the secondary endpoints. We will present our findings during the MGFA Scientific Session at the American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM) Annual Meeting on November 1, 2023, in Arizona. Preparation of a primary manuscript is underway with a plan for publication later this year.
Next, we are looking at ways to automate analysis of the videos through image processing and how this compares to other outcome measures and the expert raters. We are also building an app to collect data asynchronously from MG patients at home to be centrally analyzed and rated through image and audio processing.
The Myasthenia Gravis Rare Disease Network (MGNet) is part of the Rare Diseases Clinical Research Network (RDCRN), which is funded by the National Institutes of Health (NIH) and led by the National Center for Advancing Translational Sciences (NCATS) through its Division of Rare Diseases Research Innovation (DRDRI). MGNet is funded under grant number U54NS115054 as a collaboration between NCATS and the National Institute of Neurological Disorders and Stroke (NINDS).