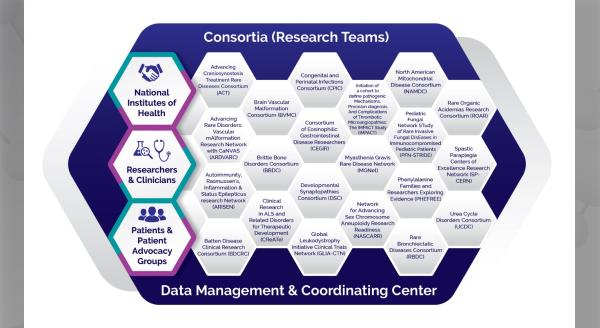
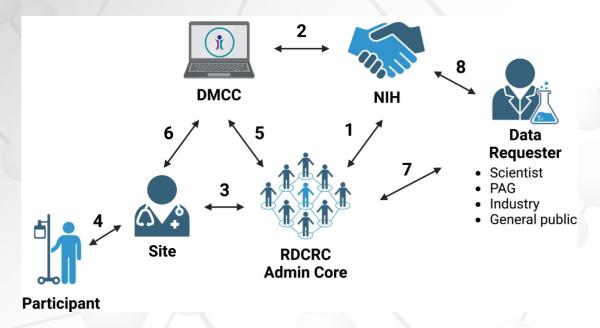
Prior to 2014, investigators researching eosinophilic gastrointestinal disorders (EGIDs) primarily collaborated on a regional basis with limited communications within or between centers. The Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR, https://www.rarediseasesnetwork.org/cms/cegir, U54 AI117804) was established in 2014 to overcome these limitations and facilitate research on EGIDs and change the paradigm of collaborative efforts for EGIDs.
Difficulties in Diagnosis
EGIDs are chronic, inflammatory conditions affecting various segments of the gastrointestinal tract of children and adults. The unifying feature of this group of these heterogeneous conditions is eosinophil-predominant inflammation in the gastrointestinal tract (see Figure 1). EGIDs are rare, challenging to diagnose, and significantly affect quality of life. EGIDs are named by the segment(s) of the gastrointestinal tract affected by eosinophilia and include eosinophilic esophagitis (EoE, esophagus), eosinophilic gastritis (EG, stomach), eosinophilic enteritis (EE, small intestine), eosinophilic colitis (EC, colon), and eosinophilic gastroenteritis (EGE, stomach and intestine). After diagnosis, disease remission is achieved and maintained by glucocorticoids and/or dietary therapy, depending on the condition. However, diagnosing EGIDs in itself can be a challenge for three primary reasons:
- Mucosal/epithelial eosinophilia is not pathognomonic to EGIDs, as other diseases (e.g., reflux esophagitis, parasitic infestations, rheumatologic conditions, inflammatory bowel diseases) have mucosal eosinophilia
- Histologically assessing non-esophageal EGIDs may be confounded by the presence of resident eosinophils in non-diseased gastrointestinal mucosa, especially as consensus is lacking for optimal cutoff values of normal variations in eosinophil density
- Determining the depth and involvement of pathology beyond the gastrointestinal epithelium is limited by the size and depth of endoscopically obtained biopsies
Re-envisioning Collaborations
The mission of CEGIR is to improve the lives of individuals with EGIDs through innovative research, clinical expertise, and education via collaborations between scientists, physicians, patients and professional organizations. CEGIR’s mission is addressed through three core programs:
- The Clinical Research Projects seek to define the natural history and a new therapy for EGIDs
- The Pilot/Demonstration Clinical Research Program develops and incubates novel, hypothesis-driven projects
- The Training (Career Development) Program mentors the next generation of investigators.
CEGIR comprises 12 participating clinical centers, NIH and Swiss collaborating sites, and extensive patient advocacy group involvement embodied by the efforts of the American Partnership for Eosinophilic Diseases (APFED, www.apfed.org), Campaign Urging Research for Eosinophilic Disease (CURED, www.cured.org), and the Eosinophil Family Coalition (EFC, www.eoscoalition.org). CEGIR is a multi-disciplinary, adult and pediatric clinical research program that focuses on EGIDs, the first and only one in the world and one of the 21 consortia of the Rare Diseases Clinical Research Network (RDCRN).
Clinical Research Projects
CEGIR’s inter-center collaborative infrastructure seeks to change the landscape of the lives of patients with EGIDs by discovering the natural history and identifying novel approaches to treatment. To date, the natural history of EGIDs is unknown. This information is important to inform our patients of future issues related to quality of life and is critical to drug development. Without the knowledge of what to measure as outcomes of treatment, termed patient-reported outcomes (PROs), and how to measure them, termed clinical outcome metrics (COMS), academic and industry partners are hindered in their ability to perform studies to develop new treatments. With rare diseases, identifying PROs and COMs are crucial steps because no single center alone can provide this information due to the patient numbers necessary to answer key questions. In this regard, CEGIR is dedicated to promoting collaboration among centers focused on studying EoE, EG, and EC and is collecting longitudinal clinical data and biorepository specimens in a standardized way into a centralized database. This centralized, shared information is facilitating a better understanding of the disease natural history, creating appropriate diagnostic criteria, discovering and validating predictive biomarkers, and optimizing PRO metrics and COMs that meaningfully report and quantify clinical states. Identifying these metrics is providing guidance to approval of novel therapeutic agents and optimize disease therapy. In particular, CEGIR has a therapeutic trial focused on developing a less restrictive food elimination diet and the positioning of swallowed glucocorticoids for EoE.
Pilot/Demonstration Clinical Research Program
Within the realm of the Pilot/Demonstration Clinical Research Program, CEGIR is currently:
- Identifying the microbiome associated with EGIDs
- Optimizing a novel method to monitor disease activity during diet treatments with a less invasive approach (transnasal endoscopy) compared with the current standard, which is an esophagogastroduodenoscopy (EGD) typically requiring general anesthesia, particularly in children
- Developing new treatment paradigms for EG with elemental formula
- Providing a new approach to treating EoE-related complications with losartan
This program is instrumental in supporting the start-up and evaluation of promising ideas that then lead to more extensive proposals to well-established funding agencies.
Training (Career Development) Program
CEGIR’s Training (Career Development) Program is dedicated to fostering the careers of young investigators who show great promise in becoming the next generation of leaders in EGID research and care. A core curriculum with online and face-to-face programs, specific research projects with achievable and meaningful goals, and platforms for developing academic skills are provided by CEGIR senior investigators. Five trainees who represent diverse geographic locations have been selected and are engaged in this program.
Above and Beyond
CEGIR plays an educational role by providing content and oversight to a website (www.rarediseasesnetwork.org/cms/cegir) that serves as a resource for patients, clinicians, and investigators and through having developed a RDCRN CEGIR Contact Registry for use in clinical research and education of patients. The RDCRN CEGIR Contact Registry is an online service for patients to become more knowledgeable of current CEGIR research opportunities. Although only a few years since its creation, CEGIR’s paradigm-shifting collaboration are reaping results—communication infrastructure, numerous research projects in progress, 40 collaborative publications to date, and the RDCRN CEGIR Contact Registry. The CEGIR team is composed of a diverse group of talented researchers and meets at least biweekly as a group through teleconferences and has already held its third annual meeting. The unifying model and resulting productivity and community of this consortium is itself an educational model for future multi-center consortia.
Related Articles
CEGIR – Improving research of eosinophilic gastrointestinal disorders with the patient perspective
References
- Katherine Cheng, Sandeep K. Gupta, Susanna Kantor, Jonathan Kuhl, Seema Aceves, Peter Bonis, Kelly Capocelli, Christina L. Carpenter, Mirna Chehade, Margaret Collins, Evan S. Dellon, Gary W. Falk, Rashmi Gopal-Srivastava, Nirmala Gonsalves, Ikuo Hirano, John Leung, Jeffrey P. Krischer, Vincent Mukkada, Alain Schoepfer, Jonathan Spergel, Alex Straumann, Glenn T. Furuta, and Marc E. Rothenberg. Creating a multi-center rare disease consortium – the consortium of eosinophilic gastrointestinal disease researchers. Translational Science of Rare Diseases; 2017; 2(3-4):141-155.
- Strandburg KJ, Bechtold S. The Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR): An Emerging Knowledge Consortium. In: Stranburg KJ, Frischmann BM, Madison M, eds. Governing Medical Knowledge Commons. Cambridge, United Kingdom: Cambridge University Press; 2017:390-420.