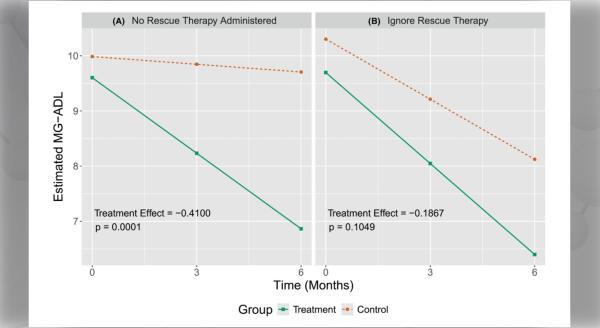
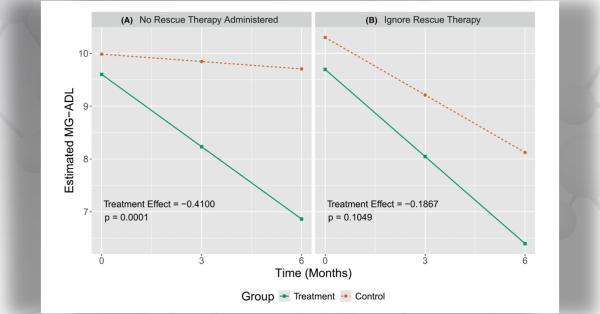
Urea cycle disorders (UCDs) are a group of inherited, metabolic disorders characterized by hyperammonemia (high blood ammonia levels). Accumulation of ammonia is toxic to the nervous system, resulting in potentially life-threatening neurological symptoms.
To learn more about the natural history of individuals with UCDs, the Urea Cycle Disorders Consortium (UCDC) is conducting a “Longitudinal Study of Urea Cycle Disorders.” The team is investigating how people with a UCD grow and develop over time and how often they get sick.
Here, lead investigators Susan Berry, MD, and Andrea Gropman, MD, share more about the study, while UCD family member Amanda Sonntag shares what it’s like to participate.
What makes this study unique?
Dr. Berry: The UCDC longitudinal study is unique in several ways. It is the longest-running sustained natural history study of any group of inherited metabolic disorders, beginning operation when the consortium was initiated in 2003 and continuing to the present. This allows us to have the opportunity to answer questions regarding care on behalf of the investigators and families who have been incredibly committed and generous in sharing their participation. The National Urea Cycle Disorders Foundation has played a critical role in helping to shape research questions and recruit patients.
Primarily, the focus has been on standard-of-care and documenting the progress of care improvements and changes in physical and cognitive development, but the data also includes research components, including additional neuropsychometric testing and quality of life assessments to learn more about long-term outcomes. We also collect blood and have a robust bio bank housed at the Coriell Institute for Medical Research through the NIGMS Human Genetic Cell Repository.
How did you become involved with this study, and why did you decide to join?
Sonntag: My son was diagnosed with partial ornithine transcarbamylase (OTC) deficiency when he was 20 months old (he is now 26 years old). I became involved in the study because I am a carrier for the gene, and I wanted to help further research for other UCD patients.
What have we learned about UCDs from this study?
Dr. Gropman: Disease Progression and Natural History: The longitudinal study has helped in our understanding of how UCDs progress over time. Tracking the natural history of the disease has provided insights into the variability of symptoms and complications that arise at all ages. This has led to focused study of adult transition, pregnancy, and quality of life, as well as the carrier females of OTC.
Clinical Manifestations and Symptoms: Observing patients over an extended period allows for a comprehensive analysis of the clinical manifestations and symptoms at different stages of the disease. Because our study commenced about 20 years ago, we can see the changes over time that occurred due to approval of new/better medications and the knowledge from the longitudinal study database that allowed us to improve our care. Patients are now living into adulthood, so following this cohort has identified new medical concerns as well as new strategies for management.
We have identified patterns or changes in symptoms over time that can aid in better management and treatment planning. One example of this is a study (manuscript submitted) of females who are carriers of OTC. The multidecade study has allowed us to evaluate whether carrier females are truly asymptomatic and if so, for how long. In other words, can an asymptomatic female become symptomatic? This is very important information for counseling and following these patients.
Treatment Efficacy and Adverse Effects: Evaluating the long-term efficacy of different treatment modalities, including medications, dietary interventions, and liver transplantation, is crucial. Assessing the occurrence and nature of adverse effects associated with treatments can help refine therapeutic approaches.
Quality of Life: Longitudinal studies can assess the impact of UCDs on the quality of life of affected individuals and their families. One of our trainees has looked at this issue specifically. Understanding how the disease affects daily activities, social interactions, and psychological well-being can inform supportive care strategies.
Genotype-Phenotype Correlations: Investigating the relationship between specific genetic mutations (genotype) and the resulting clinical presentation (phenotype) can enhance our understanding of the genetic basis of UCDs. Working with colleagues who study a yeast model of OTC has enabled us to use the genotypes of our patients in the longitudinal study to inform better phenotypic predictions using this model organism. Long-term observations may reveal genotype-specific trends in disease progression.
Cognitive and Neurological Function: UCDs can affect the central nervous system due to elevated ammonia levels. The longitudinal study has provided insights into the long-term impact on cognitive function, neurodevelopment, and neurological complications.
Biochemical Markers: Monitoring biochemical markers such as ammonia levels, amino acid profiles, and other relevant biomarkers over time can help in assessing disease severity and treatment response.
Transition to Adulthood: Tracking individuals with UCD as they transition from childhood to adulthood is crucial, as the challenges and management strategies may change during different life stages.
Emerging Therapies and Research: Longitudinal studies can serve as a platform to assess the effectiveness of emerging therapies or interventions as they become available. Contributing data to ongoing research efforts can help advance our understanding of UCDs and facilitate the development of new treatments.
What is it like to participate in this study?
Sonntag: It has been relatively easy for us to participate in the study. When my son was younger, there were many more labs and cognitive testing appointments due to the study—that was the biggest time commitment. However, his labs needed to be taken anyway for his regular appointments, so that wasn’t a problem. We were happy to participate in the study to assist the doctors and researchers in gaining further information for potential improved treatments.
How can a longitudinal study database lead to clinical trial readiness?
Dr. Gropman: A longitudinal database can play a crucial role in enhancing clinical trial readiness in several ways. Longitudinal data, collected over an extended period, provides a rich source of information about patients' health trajectories, treatment responses, and disease progression.
Patient Recruitment: Identifying and recruiting suitable participants for clinical trials can be a challenging task. Longitudinal databases can help by providing a pool of eligible patients with a specific condition or disease. Researchers can leverage historical data to identify potential participants based on specific criteria, ensuring a more targeted and efficient recruitment process.
Natural History and Disease Progression: Understanding the natural history and progression of a disease is essential for designing effective clinical trials. Longitudinal data can provide insights into how the disease evolves over time, helping researchers define appropriate endpoints and duration for clinical trials. Baseline data from the longitudinal database can inform the design of control groups and facilitate the selection of relevant outcome measures.
Endpoint Selection: Longitudinal data allow researchers to identify meaningful and clinically relevant endpoints for trials. By analyzing trends and patterns over time, investigators can choose endpoints that capture the key aspects of disease progression or treatment response.
Patient Stratification: Longitudinal databases enable the identification of subgroups within a patient population based on specific characteristics, biomarkers, or treatment responses. This information is valuable for designing stratified or personalized medicine trials, where different patient subgroups may respond differently to interventions.
Feasibility Assessment: Longitudinal data can be used to assess the feasibility of conducting a clinical trial at a particular site or within a specific patient population. Researchers can evaluate factors such as patient availability, adherence to study protocols, and the likelihood of successful recruitment based on historical data.
Real-world Evidence (RWE): Longitudinal databases contribute to the generation of real-world evidence, reflecting the outcomes and experiences of patients in routine clinical practice. Regulatory agencies increasingly recognize the value of real-world data in complementing traditional clinical trial data, especially for post-approval studies.
Identifying Candidate Biomarkers: Longitudinal data can be used to identify potential biomarkers associated with disease progression, treatment response, or adverse events. This information is valuable for designing trials that incorporate biomarker assessments and exploring targeted therapies.
Trial Design Optimization: Analyzing longitudinal data helps refine the design of clinical trials by providing insights into the variability of treatment responses, patient adherence, and potential confounding factors. Adaptive trial designs that adjust based on accumulating data can benefit from insights gained through continuous monitoring.
Regulatory Submissions: Longitudinal data may contribute to the evidence base needed for regulatory submissions. Demonstrating a deep understanding of the disease and treatment landscape, supported by longitudinal evidence, can strengthen regulatory applications. This is something that needs to be further clarified in rare disease.
Long-term Safety and Follow-up: For interventions with potential long-term effects or delayed adverse events, longitudinal databases provide a platform for monitoring safety over an extended period. Long-term follow-up data can be crucial for post-marketing surveillance and the assessment of treatment durability.
What are the successes and challenges of this study?
Dr. Berry: Our study has been very productive, increasing our overall investigational footprint by growing from five original sites to 15 sites in the United States and internationally. We have been able to successfully retain nearly 80% of our participants, despite the long duration of this study, and have generated more than 100 publications that provide information on a variety of subjects that we hope have informed patient care and decision-making for families. We have assisted in informing our National Institutes of Health (NIH) partners about strategies that can foster fruitful and appropriate interactions with industry to encourage the emergence of therapies for our families, a key desired outcome for them.
We have been challenged by the continuing difficulties in obtaining expensive and hard-to-schedule testing, primarily for neuropsychometrics and in receiving documentation of these assessments. Because of the notable interest for emerging therapies, we have ironically needed to compete with our pharma colleagues for research coordinator time occasionally, reducing our overall reach for the consortium.
What is the status of this study, and what’s next?
Dr. Berry: All good things come to an end, and our time as an NIH-supported research group is drawing to a close. We have every intention, however, of continuing this work as a group of investigators. We have nearly 1,000 enrolled subjects, and we plan to continue enrollment. We particularly are interested in trying to receive information about the rarest types of UCDs. To increase our efficiency, we are reviewing our current data collection to determine what remain the key elements for longitudinal assessment and facilitating continuing engagement with enrolled subjects. We are also hard at work on further publication using our accumulated data.
How could this study impact your life?
Sonntag: The hope is maybe someday there will be a cure for all UCDs.
The Urea Cycle Disorders Consortium (UCDC) is part of the Rare Diseases Clinical Research Network (RDCRN), which is funded by the National Institutes of Health (NIH) and led by the National Center for Advancing Translational Sciences (NCATS) through its Division of Rare Diseases Research Innovation (DRDRI). UCDC is funded under grant number U54HD061221 as a collaboration between NCATS, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).