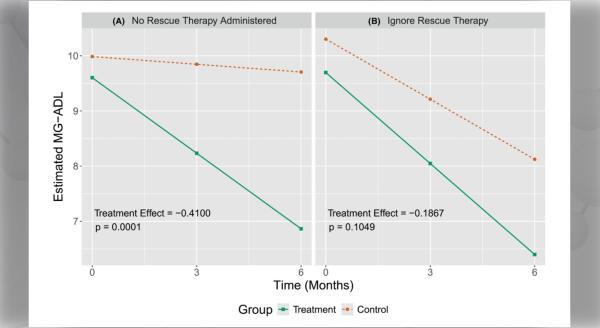
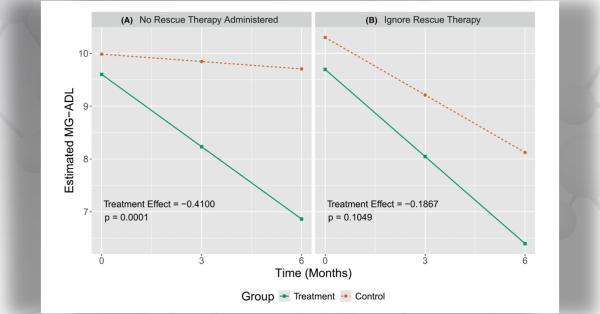
The objective of this Notice of Funding Opportunity (NOFO) is to invite new and renewal applications for the Rare Diseases Clinical Research Consortia (RDCRC) that comprise the Rare Diseases Clinical Research Network (RDCRN).
What is the RDCRN?
The RDCRN was established by The Rare Diseases Act of 2002 (Public Law 107-280), which directed the National Institutes of Health (NIH) to support "Rare Disease Regional Centers of Excellence" for clinical research, career enhancement, and demonstration of diagnostic, prevention, control, and treatment methods for rare diseases. The RDCRN has been continually funded through competitive grant cycles every five years since 2003.
What are the RDCRCs?
The RDCRCs are intended to advance and improve diagnosis, management, and treatment of numerous, diverse rare diseases through highly collaborative, multi-site, patient-centric, translational, and clinical research. Special emphasis will be placed on the early and timely identification of individuals with rare diseases and clinical trial readiness.
What qualifies as a rare disease for the RDCRN?
In this NOFO, a rare disease is defined as a condition affecting fewer than 200,000 people in the US, as defined by the Rare Diseases Act of 2002 (Public Law 107-280).
How many rare diseases must be represented in the application?
Each RDCRC application must indicate at least three different rare diseases that may share, but are not limited to, common pathways/mechanisms of action/organ system, and may be defined as:
- Conditions — a particular state of being that limits/restricts something else.
- Disorders — abnormal physical or mental conditions or ailments.
- Syndromes — a group of symptoms that occur together, or a condition characterized by a set of associated symptoms.
- Diseases — a disorder of structure or function that affects a specific location and is not simply a result of physical injury.
What is “clinical trial readiness"?
For this NOFO, clinical trial readiness is the state of having validated clinical research tools and sufficient knowledge of disease natural history to design efficient clinical trials. Validated clinical research tools can include biomarkers or clinical outcome assessment measures that are fit-for-purpose within a defined context of use relevant to the clinical trials. Knowledge of disease natural history necessary for clinical trial design can include, but is not limited to, characteristics for stratification or determining inclusion and exclusion criteria, the stage of disease progression that may be responsive to treatment, and data needed for determining sample size through power calculations.
To learn more:
- Read the full NOFO and apply.
- Read more about RDCRN applicant information.
- Please email NCATS_RDCRN@mail.nih.gov with any questions.
The Rare Diseases Clinical Research Network (RDCRN) is funded by the National Institutes of Health (NIH) and led by the National Center for Advancing Translational Sciences (NCATS) through its Division of Rare Diseases Research Innovation (DRDRI).